Abstract
The extracellular matrix (ECM) is an important regulator of angiogenesis and vascular remodeling. We showed previously that angiogenic capillaries in the developing CNS express high levels of fibronectin and its receptor α5β1 integrin, and that this expression is developmentally downregulated. As cerebral hypoxia leads to an angiogenic response, we sought to determine whether angiogenic vessels in the adult CNS re-express fibronectin and the α5β1 integrin. Ten-week old mice were subject to hypobaric hypoxia for 0, 4, 7 and 14 days, and fibronectin/integrin expression examined. Fibronectin and the α5 integrin subunit were strongly upregulated on capillaries in the hypoxic CNS, with the effect maximal at the earliest time point examined (4 days). Immunofluorescent studies demonstrated that the α5 integrin was expressed by angiogenic endothelial cells. In light of the defined angiogenic role for fibronectin in other systems, this work suggests that induction of fibronectin-α5β1 integrin expression may be an important molecular switch driving angiogenesis in the hypoxic CNS.
Keywords: CNS, Angiogenesis, Hypoxia, Capillary, Extracellular matrix (ECM), Fibronectin, α5β1 integrin, MECA-32
Introduction
The growth of new capillaries (angiogenesis) occurs in the central nervous system (CNS), not just during development (Marin-Padilla, 1985; Plate et al., 1994; Plate, 1999), but also in a number of conditions within the adult CNS, including brain tumors (Plate and Risau, 1995; Bello et al., 2004; Jansen et al., 2004), arterio-venous malformations (AVMs) (Rothbart et al., 1996; Uranashi et al., 2001), and cerebral ischemia (Garcia et al., 1971). Analysis of post-mortem tissue of stroke patients has shown that cerebral ischemia stimulates the growth of new capillaries in areas surrounding the ischemic core, also known as the ischemic penumbra (Krupinski et al., 1994). This finding has been confirmed using animal models of stroke (Chen et al., 1994; Wei et al., 2001; Hayashi et al., 2003; Wang et al., 2004).
Angiogenesis is promoted by growth factors including vascular endothelial growth factor (VEGF) (Millauer et al., 1993; Ferrara et al., 1996), basic fibroblast growth factor (bFGF) (Klein et al., 1997), transforming growth factor beta 1 (TGF-β1) (Roberts et al., 1986; Madri et al., 1992), and the angiopoieteins (Sato et al., 1995; Maisonpierre et al., 1997). In addition to growth factors, the extracellular matrix (ECM) also plays an essential role in regulating angiogenesis and vascular remodeling (Stromblad and Cheresh, 1996; Eliceiri and Cheresh, 2001; Iivanainen et al., 2003; Stupack and Cheresh, 2003). Null mutations in the fibronectin gene (George et al., 1993) or in several of the ECM receptors integrins (Yang et al., 1993, 1995; Bader et al., 1998; Zhu et al., 2002), result in defective blood vessel formation. We have shown previously that capillary maturation in the postnatal CNS is associated with a developmental switch in the expression of ECM proteins and endothelial cell β1 integrins (Milner and Campbell, 2002). During developmental angiogenesis, cerebral capillaries express high levels of fibronectin and the fibronectin receptor α5β1 integrin. Vessel maturation is then associated with loss of fibronectin and the α5β1 integrin, and upregulation of laminin and the laminin receptors, α1β1 and α6β1. Taken together with the essential role for fibronectin in developmental angiogenesis (George et al., 1993), and the finding that fibronectin is a potent promoter of brain endothelial cell survival and proliferation (Wang and Milner, 2006), this work implies that during development, fibronectin promotes cerebral angiogenesis, via the α5β1 integrin. In light of this, we have become interested in the notion that the fibronectin-α5β1 integrin interaction may also drive angiogenesis in the adult CNS.
In the current study we have begun to address this question by examining the influence of cerebral hypoxia on fibronectin and α5β1 integrin expression in cerebral blood vessels in the adult mouse. When exposed to chronic mild hypoxia, blood vessels in the CNS show a pronounced and robust angiogenic response that results in increased capillary density (LaManna et al., 1992, 1998, 2004). The mechanisms underlying this change in capillary density have not been completely elucidated, though it has been demonstrated that cerebral hypoxia stimulates increased expression of hypoxia inducible factor-1 (HIF-1α) (Chavez et al., 2000), vascular endothelial growth factor (VEGF) (Kuo et al., 1999) and angiopoietin-2 (Pichiule and LaManna, 2002). Here, we have used immunohistochemistry, dual immunofluorescence and western blotting to examine the influence of hypobaric hypoxia on fibronectin and α5β1 integrin expression on angiogenic blood vessels in the adult mouse CNS.
Results
Cerebral hypoxia promotes increased capillary density in the CNS
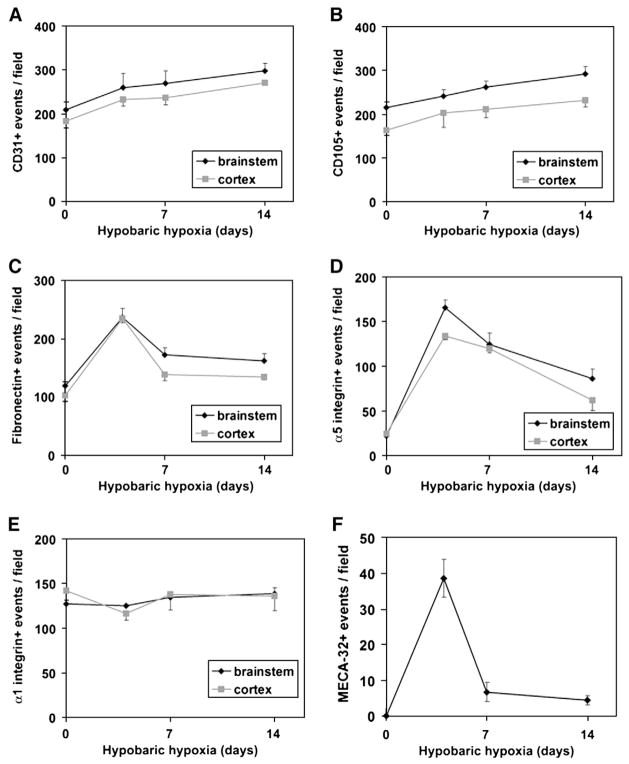
Mice or rats subject to hypobaric hypoxia show an increased capillary density within the CNS as a result of vascular remodeling (LaManna et al., 1992, 1998, 2004). In the current study we used a mouse model of hypobaric hypoxia to examine the influence of cerebral hypoxia on fibronectin and α5β1 integrin expression on angiogenic blood vessels in the CNS. Four groups of animals were studied: normoxic (control), and animals subject to hypobaric (0.4 atmospheric pressure) hypoxia (equivalent to 8% O2) for 4, 7 or 14 days. Capillaries were identified using a monoclonal antibody specific for CD31 (PECAM-1), and the capillary density quantified by counting the number of CD31-positive capillaries per field of view at ×10 magnification. As shown in Figs. 1 and 2A, over the course of 14 days, cerebral hypoxia induced an increase in capillary density in two different regions of the CNS examined, the brainstem and cerebral cortex. Fourteen-day hypoxia increased the number of CD31-positive vessels per field of view, from 208.9± 17.3 to 297.6±18.2 in the brainstem (p<0.05) and from 183.2± 16.9 to 270.8±3.8 in the cerebral cortex (p<0.05). Similar results were obtained when capillaries were identified using another monoclonal antibody specific for the endothelial cell antigen, endoglin (CD105), with 14-day hypoxia increasing the number of CD105-positive vessels per field of view, from 215.6±12.5 to 292.8±17.5 in the brainstem (p<0.05) and from 163.2±11.0 to 231.8±15.1 in the cerebral cortex (p<0.05) (Fig. 2B). These results are entirely consistent with previous observations (LaManna et al., 1992, 1998, 2004; Kanaan et al., 2006).
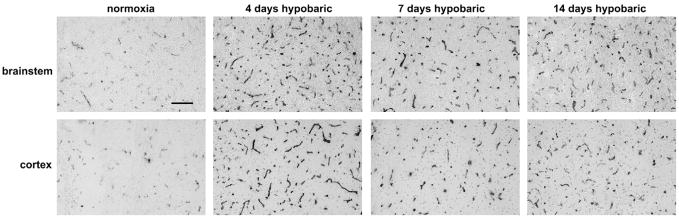
Fig. 1.
Hypoxic-induced changes in brain capillary density, as assessed by CD31 immunohistochemistry. Frozen sections of brainstem or cerebral cortex taken from mice exposed to normoxic conditions or 4, 7 or 14-day hypobaric hypoxia were immunostained with a CD31 monoclonal antibody as described in Experimental methods. Scale bar=100 μm. Note that over a 14-day period, hypobaric hypoxia induced a gradual increase in capillary density in both regions examined.
Fig. 2.
Quantification of hypoxic-induced changes in brain capillary density and vascular expression of fibronectin, integrins and the MECA-32 antigen in the brainstem and cerebral cortex. Frozen brain sections of mice exposed to normoxia, or 4, 7 or 14-day hypobaric hypoxia were examined for the number of structures that stained positive for CD31 (A), CD105 (B), fibronectin (C), α5 integrin (D), α1 integrin (E) or the MECA-32 antigen (F). Each experiment was performed with three different animals for each condition, and the results expressed as the mean±SD of the number of capillaries positive for each antigen per field of view. Note that hypobaric hypoxia promoted an increase in the capillary density in brainstem and cerebral cortex as indicated by the increased density of CD31 and CD105-positive events. In addition, hypoxia promoted an increased capillary expression of fibronectin, α5 integrin and MECA-32 antigen in both areas of the CNS, with the greatest effect being at the earliest time point examined (4 days). Expression levels of the α1 integrin were not significantly altered.
Cerebral hypoxia promotes cerebrovascular expression of fibronectin and its cell surface receptor, the α5β1 integrin
The expression of fibronectin in brain tissue was characterized using an anti-fibronectin polyclonal antibody. As shown in Fig. 3, while fibronectin was only weakly expressed by a sparse number of capillaries in the normoxic CNS, hypoxia strongly increased the expression of fibronectin on capillaries throughout the CNS. Examination of the staining intensity revealed that fibronectin expression on blood vessels was highest at day 4, and then declined thereafter. Quantification showed that in both areas of the CNS examined (brainstem and cerebral cortex), the number of fibronectin-positive capillaries was highest at the earliest time point examined (day 4) and then declined at later time points (Fig. 2C). Four-day hypoxia increased the number of fibronectin-positive vessels per field of view, from 119.2±7.1 to 237±15.0 in the brainstem (p<0.02) and from 102.7±10.2 to 234.8±7.7 in the cerebral cortex (p<0.01). Total levels of fibronectin expression within the CNS were also examined by western blotting of whole brain lysates. This showed that hypoxia increased fibronectin levels in the CNS, and that this effect was maximal at the 4-day time point, the earliest time point examined (see Fig. 4). After 4-day hypoxia the level of fibronectin protein was increased 7.3± 2.1-fold compared to normoxic conditions (p<0.05).
Fig. 3.
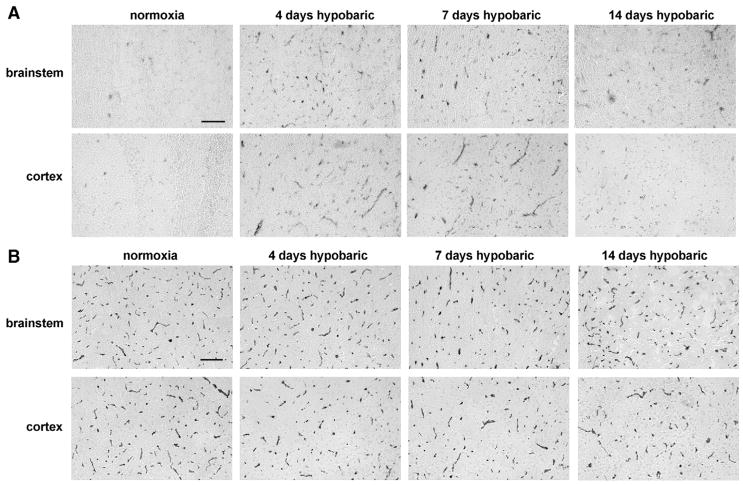
Hypoxic-induced alterations in brain capillary expression of fibronectin. Frozen sections of brainstem or cerebral cortex taken from mice exposed to normoxic conditions or 4, 7 or 14-day hypobaric hypoxia were immunostained with a polyclonal anti-fibronectin antibody, as described in Experimental methods. Scale bar=100 μm. Note that hypobaric hypoxia induced a strong increase in vascular expression of fibronectin in both brainstem and cerebral cortex, with the strongest effect being apparent by day 4 hypobaric hypoxia.
Fig. 4.
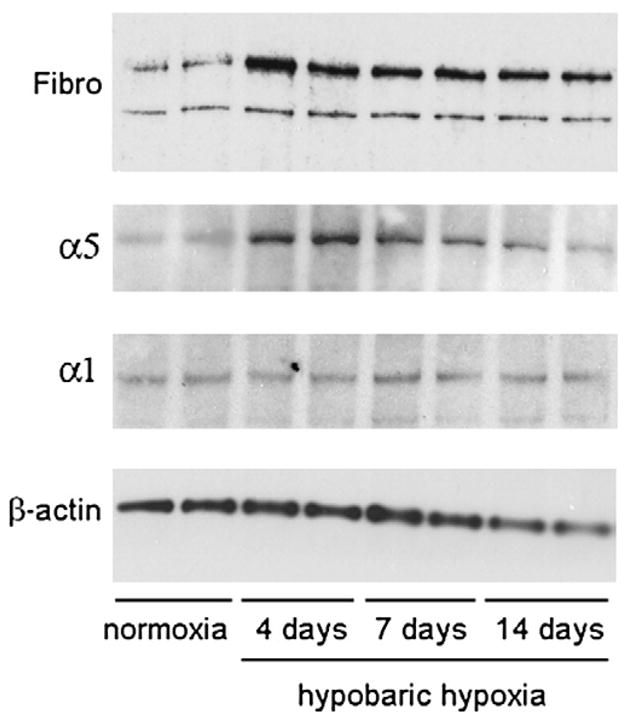
Western blot analysis of hypoxic-induced changes in brain expression of fibronectin and the α1 and α5 integrin subunits. Protein lysates obtained from the brains of mice exposed to normoxia or 4, 7 or 14-day hypobaric hypoxia were resolved by SDS-PAGE on 8% gels, transferred to nitrocellulose membranes, and protein bands revealed as described in Experimental methods. Note that hypobaric hypoxia promoted increased expression of fibronectin and the α5 integrin subunit, with the greatest effect being at 4-day hypoxia. Hypobaric hypoxia had no effect on expression of the α1 integrin subunit.
Expression of the α5β1 integrin on cerebral capillaries was examined using a monoclonal antibody against the α5 integrin subunit. Consistent with previous studies (Milner and Campbell, 2002, 2006), very few α5 integrin-positive vessels were detected within the normoxic adult CNS (Fig. 5A). However, hypoxia triggered a marked induction of the α5 integrin subunit on capillaries throughout the CNS, with the number of α5 integrin-positive capillaries being increased after days 4 hypoxia by approximately 7-fold (from 22.3±1.9 to 165.5±8.6 vessels per field of view, p<0.01), and 6-fold (from 24.9±3.3 to 134.1±4.3 vessels per field of view, p<0.01), in the brainstem and cerebral cortex, respectively. At subsequent time points the number of α5 integrin-positive capillaries gradually declined (Figs. 2D and 5A). Western blotting of whole brain lysates confirmed that expression of the α5 integrin subunit was increased by hypoxia, reaching a peak at the earliest time point studied (4 days) and then declining at later time points (Fig. 4). After 4-day hypoxia the level of α5 integrin protein was increased 5.2±0.7-fold compared to normoxic conditions (p<0.01). To exclude the possibility that hypobaric hypoxia may stimulate a generalized increased expression of all integrin subunits in cerebral blood vessels, we also examined expression of the α1 subunit, which is expressed by endothelial cells within established cerebral blood vessels. In contrast to the hypoxic upregulation of the α5 integrin subunit, the number of α1 integrin-positive blood vessels was not significantly increased by hypobaric hypoxia, as demonstrated by immunohistochemistry (Fig. 5B), quantification of absolute numbers of α1-positive vessels (Fig. 2E) or western blotting (Fig. 4). Interestingly, while the capillary density increased over the 14-day period of hypoxia, the number of α1 integrin-positive vessels did not change, so that the proportion of capillaries expressing the α1 integrin subunit actually decreased by day 4 (by 21.1±4.5% in the brainstem, p<0.02, and by 35.1±4.6% in the cerebral cortex, p<0.01). In contrast, the fraction of capillaries expressing the α5 integrin subunit showed a marked increase by day 4 and declined at later time points.
Fig. 5.
The influence of hypobaric hypoxia on brain capillary expression of the α5 (A) and α1 (B) integrin subunits. Frozen sections of brainstem or cerebral cortex taken from mice exposed to normoxic conditions or 4, 7 or 14-day hypobaric hypoxia were immunostained with α5 or α1 integrin-specific antibodies, as described in Experimental methods. Scale bar=100 μm. Note that hypobaric hypoxia induced a robust increase in vascular expression of the α5 integrin subunit in both brainstem and cerebral cortex, with the strongest effect being apparent by day 4 hypoxia. In contrast, hypoxia did not significantly alter the number of blood vessels expressing the α1 integrin subunit.
The MECA-32 antigen, not expressed by mature brain endothelium, is re-expressed on a sub-population of capillaries in the hypoxic CNS
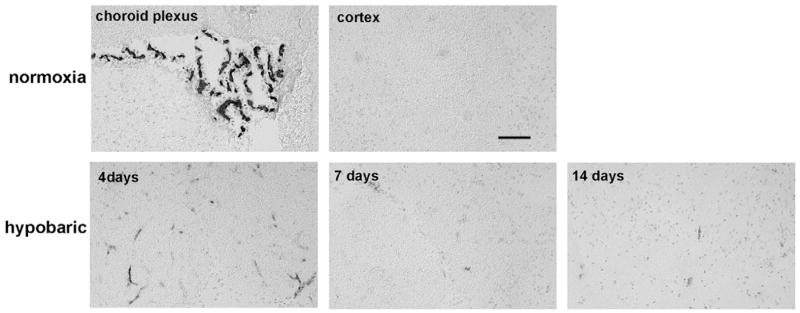
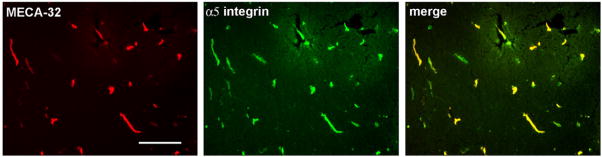
Previous studies have shown that the MECA-32 (mouse endothelial cell antigen) monoclonal antibody labels immature mouse cerebral capillaries during early development, but fails to label cerebral capillaries after the blood-brain barrier begins to form, around mouse embryonic at day 17 (Hallman et al., 1995). Based on this we reasoned that capillaries undergoing angiogenic remodeling in the hypoxic CNS might re-express the MECA-32 antigen. As shown in Fig. 6, in the normoxic adult CNS, the MECA-32 antigen was expressed only by endothelial cells within the choroid plexus (which lack the relatively impermeable BBB phenotype), but was not expressed by endothelial cells within the CNS parenchyma. In contrast, 4 days of hypobaric hypoxia induced significant expression of MECA-32 on capillaries within the CNS parenchyma (38.6±2.4 positive vessels per field of view, compared with no staining at all under normoxic conditions, p<0.01). Interestingly, as for the temporal profiles of fibronectin and α5 integrin, the number of MECA-32-positive capillaries was also highest at the earliest time point examined (day 4), declining thereafter. As the MECA-32 antigen is expressed only by immature cerebral endothelial cells (Hallman et al., 1995), this provides a useful marker to identify angiogenic immature endothelial cells within the adult CNS. Using this approach, we next examined whether hypoxia induces α5 integrin expression on blood vessels in a generalized manner, or whether α5 is specifically induced on angiogenic capillaries. Dual-color immunofluorescence was used to define the expression of the MECA-32 antigen and α5 integrin within the CNS of mice exposed to 4-day hypobaric hypoxia. As shown in Fig. 7, capillaries that stained positive for the MECA-32 antigen, also stained positive for the α5 integrin subunit, confirming that the α5 integrin subunit is expressed by angiogenic endothelial cells within the hypoxic CNS.
Fig. 6.
Hypoxic induction of the MECA-32 antigen on cerebral endothelial cells. Frozen sections of cerebral cortex taken from mice exposed to normoxic conditions or 4, 7 or 14-day hypobaric hypoxia were immunostained with the MECA-32 monoclonal antibody, as described in Experimental methods. Scale bar=100 μm. Note that in the normoxic CNS, the MECA-32 antigen was expressed only by endothelial cells within the choroid plexus, but not by endothelial cells within the cerebral parenchyma. In contrast, 4 days of hypobaric hypoxia induced a marked expression of the MECA-32 antigen on blood vessels throughout the cerebral parenchyma, with the strongest effect apparent after 4-day hypoxia, and declining thereafter.
Fig. 7.
Expression of the α5 integrin subunit on angiogenic capillaries in the hypoxic CNS. Dual-color immunofluorescence was performed on frozen sections of cerebral cortex taken from mice exposed to 4-day hypobaric hypoxia, using monoclonal antibodies specific for the MECA-32 antigen (Cy-3) or the α5 integrin subunit (FITC), as described in Experimental methods. Scale bar=50 μm. Note that capillaries that stained positive for the MECA-32 antigen, also stained positive for the α5 integrin subunit, confirming that the α5 integrin subunit is expressed by angiogenic endothelial cells within the hypoxic CNS.
Discussion
We previously demonstrated that during CNS development, angiogenic capillaries show high expression of the α5β1 integrin and its ECM ligand fibronectin, and that this expression is switched off with blood vessel maturation (Milner and Campbell, 2002). In light of this finding and the work of others defining an important angiogenic role for the α5β1 integrin in systems outside the CNS (Yang et al., 1993; Kim et al., 2000a, b; Taverna and Hynes, 2001; Francis et al., 2002), we wanted to examine whether the developmental expression profile of the α5β1 integrin is recapitulated during angiogenesis in the adult CNS. In the current study we examined this question in a mouse model of cerebral hypoxia, in which mice are exposed to hypobaric hypoxia for up to 2 weeks, during which time vascular remodeling and increased capillary growth occur (LaManna et al., 1992, 1998, 2004; Kanaan et al., 2006). Our fundamental observation is that cerebral hypoxia induced a marked transient increase in expression of fibronectin and the α5β1 integrin by cerebral capillaries.
Effect of cerebral hypoxia on capillary density in the CNS
Several studies have documented an increased capillary density in the CNS of mice or rats exposed to hypobaric hypoxia (LaManna et al., 1992, 1998, 2004; Kanaan et al., 2006). Previous studies in mice used the endothelial cell marker, GLUT-1 to quantify capillary density (Kanaan et al., 2006). In the current study we used two different endothelial cell markers, CD31 (PECAM-1) and CD105 (endoglin), which both showed that hypobaric hypoxia led to an increased capillary density in both areas of the CNS examined, the brainstem and the cerebral cortex.
Hypoxic induction of the α5β1 integrin on cerebral capillaries
It is well established that hypoxia leads to active vascular remodeling, and increased capillary density, not just in the CNS (LaManna et al., 1992, 1998, 2004; Kanaan et al., 2006), but also in other tissues including cardiac (Sasaki et al., 2001, 2002) and skeletal (Hudlicka et al., 2002; Däpp et al., 2006) muscle. As ECM–integrin interactions play a fundamental role in regulating angiogenesis and vascular remodeling (Stromblad and Cheresh, 1996; Eliceiri and Cheresh, 2001; Iivanainen et al., 2003; Stupack and Cheresh, 2003), it seems highly likely that changes in ECM–integrin expression may be, at least in part, responsible for the vascular response to hypoxia. During CNS development, cerebral capillaries show a major switch from fibronectin signaling pathways (high expression of fibronectin and the fibronectin receptor α5β1 integrin) during the angiogenic phase to laminin signaling pathways (high expression of laminin and the laminin receptors α1β1 and α6β1) in mature cerebral capillaries (Milner and Campbell, 2002). In light of this switch, it was important to examine whether a similar angiogenic switch also occurs in the adult CNS in conditions that promote cerebral angiogenesis, such as hypoxia. The work presented here clearly shows that in the adult mouse CNS, cerebral hypoxia promotes increased expression of the α5β1 integrin and fibronectin on cerebral capillaries. This is consistent with our recent observation that when brain endothelial cells are exposed to hypoxia in vitro, they show increased expression of the α5 and β1 integrin subunits (Milner et al., 2007b). Taken together, these findings are important for several reasons. First, to our knowledge this is the first in vivo evidence that hypoxia regulates integrin expression by vascular cells in the CNS. Second, this work shows that the developmental program defining a role for the α5β1 integrin in cerebral angiogenesis is recapitulated in the adult CNS, suggesting that fibronectin-α5β1 integrin interactions represent a fundamental pro-angiogenic pathway in the CNS. Third, these observations have potential therapeutic implications, as manipulation of the expression or signaling of the α5β1 integrin may represent a promising approach to enhance cerebral angiogenesis in the adult CNS, either before, or immediately after ischemic stroke.
Evidence from outside the CNS supports the concept that the α5β1 integrin is an important promoter of angiogenesis. Mice null for the α5 integrin show major vascular defects (Yang et al., 1993), and embryonic stem (ES) cells lacking the α5 integrin subunit show deficient blood vessel formation (Taverna and Hynes, 2001; Francis et al., 2002). In addition, induction of the α5β1 integrin has been described on tumor-associated angiogenic blood vessels in mouse and chicken, and functional blockade of this integrin halts angiogenesis and tumor growth (Kim et al., 2000a, b). Extra support comes from in vitro studies showing that fibronectin is a strong promoter of endothelial cell survival, proliferation and migration, aspects of cell behavior intrinsic to angiogenesis (Ingber and Folkman, 1988; McIntosh et al., 1988; Kirkpatrick et al., 1990; Ingber, 1992). Recently we showed that fibronectin is extremely potent at promoting brain endothelial cell survival and proliferation, and that these effects are mediated via the α5β1 and αvβ3 integrins (Wang and Milner, 2006).
Increased expression of fibronectin in capillaries following cerebral hypoxia
Hypoxia stimulated a large increase in fibronectin expression within cerebral capillaries. This is consistent with reactivation of the developmental program, in which fibronectin is expressed at high levels by capillaries undergoing angiogenesis (Marin-Padilla, 1985; Risau and Lemmon, 1988; Milner and Campbell, 2002). Many studies have described fibronectin upregulation on angiogenic capillaries in a number of situations, including skin development (Tonnesen et al., 1985), wound-healing (Clark et al., 1982a, b), and tumor-induced angiogenesis (Castellani et al., 1994; Neri et al., 1997). In tumor-induced angiogenic capillaries, a specific alternatively spliced form of fibronectin is induced, containing the EIIIB domain (Neri et al., 1997). This observation is currently being exploited in the clinic, both to monitor the progression of metastatic tumor growth, and to target tumor-induced vessels. In the current study, we used a polyclonal antibody that does not differentiate between the different spliced forms of fibronectin, but in future studies we will determine if the EIIIB form of fibronectin is also specifically upregulated in angiogenic cerebral capillaries in response to hypoxia.
Temporal expression pattern of fibronectin, α5 integrin and MECA-32
One observation to emerge from our study was that the physiological response to cerebral hypoxia was strongest at the earliest time point studied, 4 days. While the overall capillary density as measured by CD31 and CD105, continued to increase over the 14-day time course, the expression profiles of fibronectin, α5 integrin and the angiogenic marker MECA-32 were maximal by day 4, and declined thereafter. This timing is similar to a previous report showing that VEGF expression in the hypoxic CNS peaks at day 2 and falls thereafter (Kuo et al., 1999). It also implies that the fibronectin-α5β1 integrin interaction is required for the early stages of angiogenic remodeling, which is in keeping with evidence that fibronectin promotes aspects of endothelial cell behavior intrinsic to the early stages of angiogenesis, such as endothelial cell proliferation and migration, but plays less of a role in the later stages of angiogenesis, such as endothelial cell differentiation and vessel stabilization.
In light of the finding that the MECA-32 antigen is expressed by brain endothelial cells early in development, but then lost with BBB differentiation, it has been suggested that the MECA-32 antibody identifies immature endothelial cells (Hallman et al., 1995). In the current study, capillary expression of the MECA-32 antigen was highest after 4 days of hypobaric hypoxia, and then disappeared at later time points. This shows that the maximal response to hypoxia occurs in the first few days, during which time endothelial cells de-differentiate and express the immature marker MECA-32, but at later time points most endothelial cells have matured and switched off MECA-32 expression. On this basis, MECA-32 provides a valuable marker to identify angiogenic immature endothelial cells within the adult CNS. Employing this approach, we demonstrated that the α5 integrin subunit is expressed specifically by angiogenic endothelial cells within the hypoxic CNS.
Two other studies have described re-expression of the MECA-32 antigen on endothelial cells in the adult brain during neuroinflammatory conditions (Engelhardt et al., 1994; Sparks et al., 2000). In one of these, injection of Corynebacterium parvum induced endothelial expression of ICAM-1 and VCAM-1 on all vessels throughout the CNS, but MECA-32 expression on endothelial cells only at the center of the inflammatory infiltrate (Engelhardt et al., 1994). In the other study we showed that hypobaric hypoxia stimulates activation of cerebral endothelial cells, as indicated by increased expression of ICAM-1, E-selectin and Glut-1 (Dore-Duffy et al., 1999). Why the MECA-32 antigen is re-expressed on cerebral endothelial cells under neuroinflammatory conditions is unclear. One possibility is that MECA-32 expression reflects altered activation status of brain endothelial cells, akin to induction of VCAM-1 and ICAM-1, and that MECA-32 expression is part of a stress-induced early response common to hypoxia and inflammation. Alternatively, as angiogenesis often accompanies inflammation (Campbell et al., 1993; Milner and Campbell, 2006), induction of the MECA-32 antigen may represent early angiogenic events associated with inflammation.
One important question is what molecular signals trigger the hypoxic induction of fibronectin and the α5β1 integrin in cerebral capillaries? In the hypobaric hypoxia model it has been shown that cerebral hypoxia promotes increased expression of hypoxic inducible factor (HIF-1α) (Chavez et al., 2000), angiopoietin-2 (Pichiule and LaManna, 2002), and VEGF (Kuo et al., 1999). Other studies have revealed that the angiogenic growth factor TGF-β1 is induced following cerebral ischemia (Krupinski et al., 1996; Lehrman et al., 1998; Ruocco et al., 1999). Currently we are addressing this question by asking which of the well-described angiogenic growth factors or cytokines promote increased expression of the α5β1 integrin on brain capillary endothelial cells in vitro.
In summary, we have shown that in the hypobaric mouse model of cerebral hypoxia, cerebral capillaries show a marked transient increased expression of fibronectin and its receptor, the α5β1 integrin. This recapitulates the strong expression of these proteins observed during cerebral angiogenesis in development (Marin-Padilla, 1985; Risau and Lemmon, 1988; Milner and Campbell, 2002). Taken together with a defined angiogenic role for fibronectin (Clark et al., 1982b; George et al., 1993; Jiang et al., 1994; Neri et al., 1997) and the α5β1 integrin (Yang et al., 1993; Kim et al., 2000a, b; Taverna and Hynes, 2001) in systems outside the CNS, this data supports the concept that the fibronectin-α5β1 integrin interaction may be an important molecular switch driving angiogenesis in the hypoxic CNS. In the next set of studies we will investigate whether a similar induction of α5β1 integrin and fibronectin also occurs on angiogenic vessels in an animal model of ischemic stroke. While cerebral ischemia and hypobaric hypoxia are not entirely equivalent, cerebral ischemia leads to cerebral hypoxia and a marked angiogenic response (Chen et al., 1994; Wei et al., 2001; Lin et al., 2002; Hayashi et al., 2003), leading us to predict that upregulation of α5β1 integrin and fibronectin may also occur in this model. The outcome of these experiments will determine whether manipulation of the expression or signaling pathways of the α5β1 integrin will provide a potential therapeutic approach to enhance cerebral angiogenesis. If true, this could have important impact on the incidence and mortality of ischemic stroke.
Experimental methods
Animals
The studies described have been reviewed and approved by The Scripps Research Institute Institutional Animal Care and Use Committee. All animals were maintained under pathogen-free conditions in the closed breeding colony of The Scripps Research Institute (TSRI).
Hypobaric hypoxia
C57Bl/6 mice, 9–10 weeks of age, housed 4 to a cage, were placed into a hypobaric chamber maintained at a pressure of 300 Torr, (0.4 ATM, equivalent to 8% normobaric oxygen) for periods up to 21 days. Littermate controls were kept in the same room in a similar chamber under similar conditions except that they were kept at normobaric pressure for the duration of the experiment. Every few days, the chamber was returned briefly to normobaric pressure for cage cleaning and food and water replacement as needed.
Immunohistochemistry and antibodies
Immunohistochemistry was performed as described previously (Milner and Campbell, 2002) on 10 μm thick frozen sections of cold saline-perfused brains taken from C57/Bl6 mice subject to either normoxia (control) or hypobaric hypoxia for 4, 7 or 14 days. Each slide contained mouse brains representing the four different time points of hypoxia, to ensure consistent antibody incubation and chromogen exposure times across the different conditions. The following monoclonal antibodies were obtained from BD Pharmingen (La Jolla, CA): rat monoclonal antibodies reactive for the α5 integrin subunit (clone 5H10-27 (MFR5), CD31 (PECAM-1) (clone MEC13.3), CD105 (endoglin) (clone MJ7/18), clone MECA-32, hamster monoclonal antibodies reactive for the integrin subunits α1 (clone Ha31/8) and α5 (clone HMα5-1), and the isotype control antibodies, rat anti-KLH (A110-2) and hamster anti-TNP-KLH (G235-1). Rabbit polyclonal antibodies against the following proteins were used in this study: fibronectin (Sigma), α1 and α5 integrin subunits (Chemicon, Temecula, CA), and beta-actin (Neo-marker, Fremont, CA).
For the dual-color immunofluorescent study of α5 integrin and the MECA-32 antigen, tissue sections were incubated with the MECA-32 antibody overnight at 4 °C, washed then incubated with Cy3-conjugated anti-rat antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h at 37 °C, washed, then incubated with the α5 integrin-specific monoclonal antibody HMα5-1 for 2 h at 37 °C, washed, then incubated with biotinylated anti-hamster antibody for 1 h at 37 °C, washed, then incubated with streptavidin-FITC (BD Pharmingen) for 1 h at 37 °C, and finally washed thoroughly and mounted. Quantification of the number of capillaries positive for the different antigens was performed by taking images using a ×10 objective on a Zeiss Axiovert S100 microscope and Zeiss Axiocam digital camera. These images were used to determine the number of positive events within the area of this field (area=0.5734 mm2). Within each animal, several images of the brain stem and cerebral cortex were taken for each antigen, and the average number of events calculated. Each experiment was performed with three different animals per condition, and the results expressed as the mean±SD of the number of capillaries positive for each antigen per field of view. Statistical significance was assessed by using the Student’s t test, in which p<0.05 was defined as statistically significant.
Western blotting
Brains were removed from mice perfused with cold saline and homogenized in lysis buffer, consisting of PBS containing 1% NP40 (Sigma) and a cocktail of protease inhibitors (Invitrogen, Carlsbad, CA). After 30 min on ice, the homogenate was centrifuged to remove the insoluble fraction and the protein concentration of the brain lysate quantified (Bio-Rad Laboratories, Hercules, CA). 10 μg amounts of protein were mixed with non-reducing sample buffer (α1 and α5 integrins), or reducing sample buffer (fibronectin and β-actin), boiled for 5 min and analysed on 8% SDS-PAGE gels (Invitrogen). Proteins were electro-blotted for 1 h onto nitrocellulose membranes (Invitrogen), blocked for 1 h in 5% non-fat milk in PBS containing 0.1% Tween-20 (Sigma) and membranes probed for 1 h, washed, then incubated with anti-rabbit HRP conjugate (Sigma) for 1 h, before being extensively washed. Finally protein bands were visualised with the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer’s instructions. For protein quantification, gels were scanned using a Bio-Rad VersaDoc imaging system (Hercules, CA) and band intensities quantified using the NIH image program. Within each brain sample, levels of fibronectin and the α5 or α1 integrins were first normalised to the level of β-actin, and then expressed as the fold-increase over the level present within the brain of normoxic animals, as described previously (Milner et al., 2007a).
Acknowledgments
This work was supported in part by a Harry Weaver Neuroscience Scholar Award (JF 2125A1/1) from the National Multiple Sclerosis Society (RM), and by NIH grants NSO53716 (GdZ), NS38632 (JCL) and NS143627 and NS047672 (PD-D). This is manuscript number 19091 from The Scripps Research Institute.
References
- Bader B, Rayburn H, Crowley D, Hynes R. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Bello L, Giussani C, Carrabba G, Pluderi M, Costa F, Bikfalvi A. Angiogenesis and invasion in gliomas. Cancer Treat Res. 2004;117:263–284. doi: 10.1007/978-1-4419-8871-3_16. [DOI] [PubMed] [Google Scholar]
- Campbell I, Abraham C, Masliah E, Kemper P, Inglis J, Oldstone M, Mucke L. Neurologic disease induced in transgenic mice by the cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani P, Viale G, Dorcaratto A, Nicolo G, Kaczmarek J, Querze G, Zardi L. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int J Cancer. 1994;59:612–618. doi: 10.1002/ijc.2910590507. [DOI] [PubMed] [Google Scholar]
- Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxic inducible factor 1a in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89:1937–1942. doi: 10.1152/jappl.2000.89.5.1937. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chien CH, Liu HM. Correlation between angiogenesis and basic fibroblast growth factor expression in experimental brain infarct. Stroke. 1994;25:1651–1657. doi: 10.1161/01.str.25.8.1651. [DOI] [PubMed] [Google Scholar]
- Clark RAF, DellaPelle P, Manseau E, Lanigan JM, Dvorak HF, Colvin RB. Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary in growth during wound healing. J Invest Dermatol. 1982a;79:269–276. doi: 10.1111/1523-1747.ep12500076. [DOI] [PubMed] [Google Scholar]
- Clark RAF, Quinn JH, Winn HJ, Lanigan JM, Dellepella P, Colvin RB. Fibronectin is produced by blood vessels in response to injury. J Exp Med. 1982b;156:646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Däpp C, Gassmann M, Hoppeler H, Flück M. Hypoxia-induced gene activity in disused oxidative muscle. Adv Exp Med Biol. 2006;588:171–188. doi: 10.1007/978-0-387-34817-9_16. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Balabanov R, Beaumont T, Hritz MA, Harik SI, LaManna JC. Endothelial activation following prolonged hypobaric hypoxia. Microvasc Res. 1999;57:75–85. doi: 10.1006/mvre.1998.2112. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Cheresh D. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13:563–568. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Conley FK, Butcher EC. Cell adhesion molecules on vessels during neuroinflammation in the mouse central nervous system. J Neuroimmunol. 1994;51:199–208. doi: 10.1016/0165-5728(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5 beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Cox J, Hudgins W. Ultrastructure of the microvasculature in experimental cerebral infarction. Acta Neuropath. 1971;18:273–285. doi: 10.1007/BF00688441. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Hallman R, Mayer DN, Berg EL, Broermann R, Butcher EC. Novel mouse endothelial cell surface marker is suppressed during differentiation of the blood brain barrier. Dev Dyn. 1995;202:325–332. doi: 10.1002/aja.1002020402. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Milkiewicz M, Cotter MA, Brown MD. Hypoxia and expression of VEGF-A protein in relation to capillary growth in electrically stimulated rat and rabbit skeletal muscles. Exp Physiol. 2002;87:373–381. doi: 10.1113/eph8702285. [DOI] [PubMed] [Google Scholar]
- Iivanainen E, Kahari V-M, Heino J, Elenius K. Endothelial cell-matrix interactions. Microsc Res Tech. 2003;60:13–22. doi: 10.1002/jemt.10238. [DOI] [PubMed] [Google Scholar]
- Ingber D. Extracellular matrix as a solid-state regulator in angiogenesis: identification of new targets for anti-cancer therapy. Semin Cancer Biol. 1992;3:57–63. [PubMed] [Google Scholar]
- Ingber D, Folkman J. Inhibition of angiogenesis through modulation of collagen metabolism. Lab Invest. 1988;59:44–51. [PubMed] [Google Scholar]
- Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJF. Current perspectives on antiangiogenic strategies in the treatment of malignant gliomas. Brain Res Rev. 2004;45:143–163. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci. 1994;107:2499–2508. doi: 10.1242/jcs.107.9.2499. [DOI] [PubMed] [Google Scholar]
- Kanaan A, Farahani R, Douglas RM, LaManna JC, Haddad GC. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol, Regul Integr Comp Physiol. 2006;290:R1105–R1114. doi: 10.1152/ajpregu.00535.2005. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin a5b1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000a;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Harris M, Varner JA. Regulation of avb3-mediated endothelial cell migration and angiogenesis by integrin a5b1 and protein kinase A. J Biol Chem. 2000b;275:33920–33928. doi: 10.1074/jbc.M003668200. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CJ, Kampe M, Rixen H, Fischer EG, Ruchatz D, Mittermayer C. In vitro studies on the expansion of endothelial cell monolayers on components of the basement membrane. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;58:207–213. doi: 10.1007/BF02890073. [DOI] [PubMed] [Google Scholar]
- Klein S, Roghani M, Rifkin DB. Fibroblast growth factors as angiogenesis factors. EXS. 1997;79:159–192. doi: 10.1007/978-3-0348-9006-9_7. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke. 1994;25:1794–1798. doi: 10.1161/01.str.25.9.1794. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-β1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27:852–857. doi: 10.1161/01.str.27.5.852. [DOI] [PubMed] [Google Scholar]
- Kuo N-T, Benhayon D, Przybylski RJ, Martin RJ, LaManna JC. Prolonged hypoxia increases vascular endothelial growth factor mRNA and protein in adult mouse brain. J Appl Physiol. 1999;86:260–264. doi: 10.1152/jappl.1999.86.1.260. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Kuo NT, Lust WD. Hypoxia-induced brain angiogenesis. Signals and consequences. Adv Exp Med Biol. 1998;454:287–293. doi: 10.1007/978-1-4615-4863-8_34. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- Lehrman E, Kiefer R, Christensen T, Toyka KV, Zimmer J, Diemer NH, Hartung HP, Finsen B. Microglia and macrophages are major sources of locally produced transforming growth factor-beta 1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24:437–448. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lin TN, Sun SW, Cheung WM, Chang C. Dynamic changes in cerebral blood flow and angiogenesis after transient focal cerebral ischemia in rats. Evaluation with serial magnetic resonance imaging. Stroke. 2002;33:2985–2991. doi: 10.1161/01.str.0000037675.97888.9d. [DOI] [PubMed] [Google Scholar]
- Madri JA, Bell L, Merwin JR. Modulation of vascular cell behavior by transforming growth factors beta. Mol Reprod Dev. 1992;32:121–126. doi: 10.1002/mrd.1080320207. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton DL, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie-2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Early vascularization of the embryonic cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- McIntosh LC, Muckersie L, Forrester JV. Retinal capillary endothelial cells prefer different substrates for growth and migration. Tissue Cell. 1988;20:193–209. doi: 10.1016/0040-8166(88)90041-9. [DOI] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ulrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of b1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Increased expression of the beta 4 and alpha 5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci. 2006;33:429–440. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, Del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins α5β1 and αvβ5. J Immunol. 2007a;178:8158–8167. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg G, Spatz M, del Zoppo G. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2007b;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri D, Camemolla B, Nissim A, Leprini A, Querze G, Balza E, Pini A, Tarli L, Halin C, Neri P, Zardi L, Winer G. Targeting by affinity-matured recombinant fragments of an angiogenesis associated fibronectin isoform. Nat Biotechnol. 1997;15:1271–1275. doi: 10.1038/nbt1197-1271. [DOI] [PubMed] [Google Scholar]
- Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and de-adaptation to prolonged mild hypoxia. J Appl Physiol. 2002;93:1131–1139. doi: 10.1152/japplphysiol.00318.2002. [DOI] [PubMed] [Google Scholar]
- Plate KH. Mechanisms of angiogenesis in the brain. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- Plate KH, Breier G, Risau W. Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol. 1994;4:207–218. doi: 10.1111/j.1750-3639.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15:339–347. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125:441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield U, Heine I, Liotta A, Falanga J, Kehrl JH, Fauci AS. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart D, Awad IA, Lee J, Kim J, Harbaugh R, Criscuolo GR. Expression of angiogenic factors and structural proteins in central nervous system vascular malformations. Neurosurgery. 1996;38:915–924. doi: 10.1097/00006123-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Ruocco A, Nicole O, Docagne F, Ali C, Chazaliel L, Komelski S, Yablonsky F, Roussel S, MacKenzie ET, Vivien D, Buisson A. A transforming growth factor-beta antagonist unmasks the neuroprotective role of this cytokine in excitotoxic and ischemic brain injury. J Cereb Blood Flow Metab. 1999;19:1345–1353. doi: 10.1097/00004647-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Ray PS, Zhu L, Otani H, Asahara T, Maulik N. Hypoxia/reoxygenation promotes myocardial angiogenesis via an NF kappa B-dependent mechanism in a rat model of chronic myocardial infarction. J Mol Cell Cardiol. 2001;33:283–294. doi: 10.1006/jmcc.2000.1299. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Fukuda S, Otani H, Zhu L, Yamaura G, Engelman RM, Das DK, Maulik N. Hypoxic preconditioning triggers myocardial angiogenesis: a novel approach to enhance contractile functional reserve in rat with myocardial infarction. J Mol Cell Cardiol. 2002;34:335–348. doi: 10.1006/jmcc.2001.1516. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Kuo YM, Roher A, Martin T, Lukas RJ. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Ann NY Acad Sci. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Stromblad S, Cheresh DA. Integrins, angiogenesis and vascular cell survival. Chem Biol. 1996;3:881–885. doi: 10.1016/s1074-5521(96)90176-3. [DOI] [PubMed] [Google Scholar]
- Stupack DG, Cheresh D. Apoptotic cues from the extracellular matrix: regulators of angiogenesis. Oncogene. 2003;22:9022–9029. doi: 10.1038/sj.onc.1207110. [DOI] [PubMed] [Google Scholar]
- Taverna D, Hynes RO. Reduced blood vessel formation and tumor growth in alpha5-integrin-negative teratocarcinomas and embryoid bodies. Cancer Res. 2001;61:5255–5261. [PubMed] [Google Scholar]
- Tonnesen MG, Jenkins D, Siegal SL, Lee LA, Huff JC, Clark RA. Expression of fibronectin, laminin, and factor VIII-related antigen during development of the human cutaneous microvasculature. J Invest Dermatol. 1985;85:564–568. doi: 10.1111/1523-1747.ep12277410. [DOI] [PubMed] [Google Scholar]
- Uranashi R, Baev NI, Ng PY, Kim JH, Awad IA. Expression of endothelial cell angiogenesis receptors in human cerebrovascular malformations. Neurosurgery. 2001;48:359–367. doi: 10.1097/00006123-200102000-00024. [DOI] [PubMed] [Google Scholar]
- Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through a5b1 and avb3 integrins via MAP kinase signaling. J Neurochem. 2006;96:148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wei L, Erinjeri JP, Rovainen CM, Woolsey TA. Collateral growth and angiogenesis around cortical stroke. Stroke. 2001;32:2179–2184. doi: 10.1161/hs0901.094282. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in a5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Scmidt A, Reichardt LF. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]