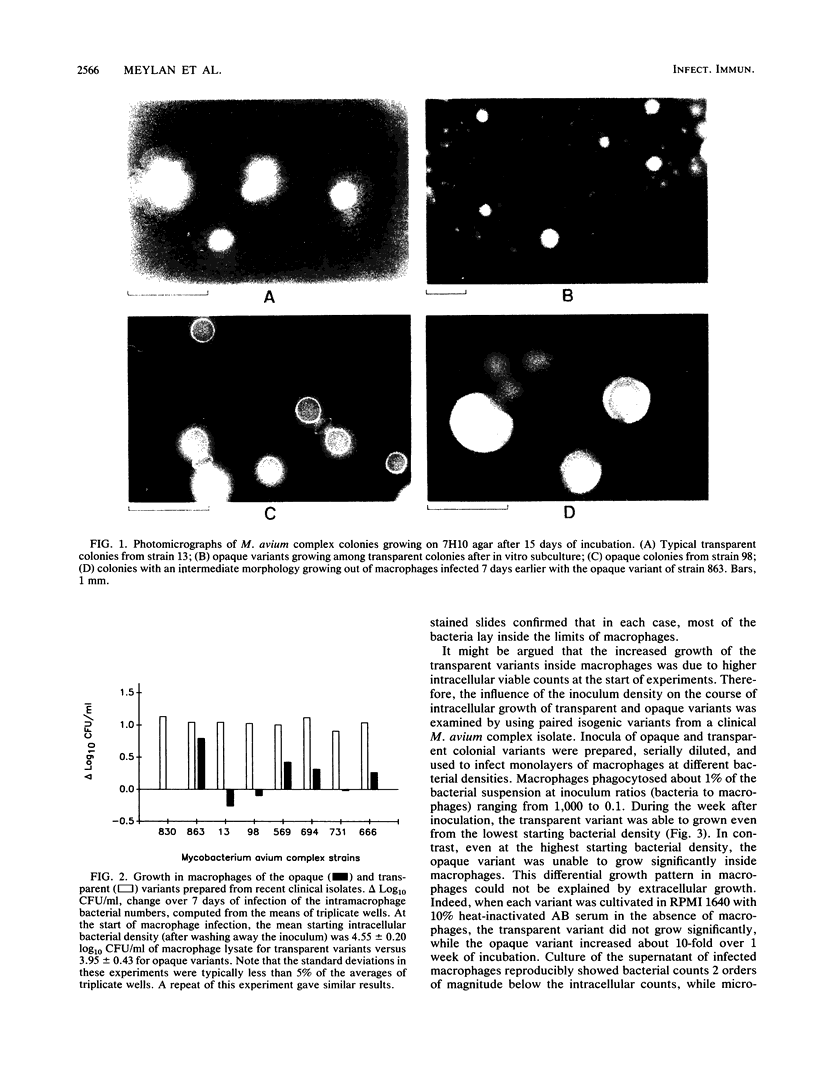
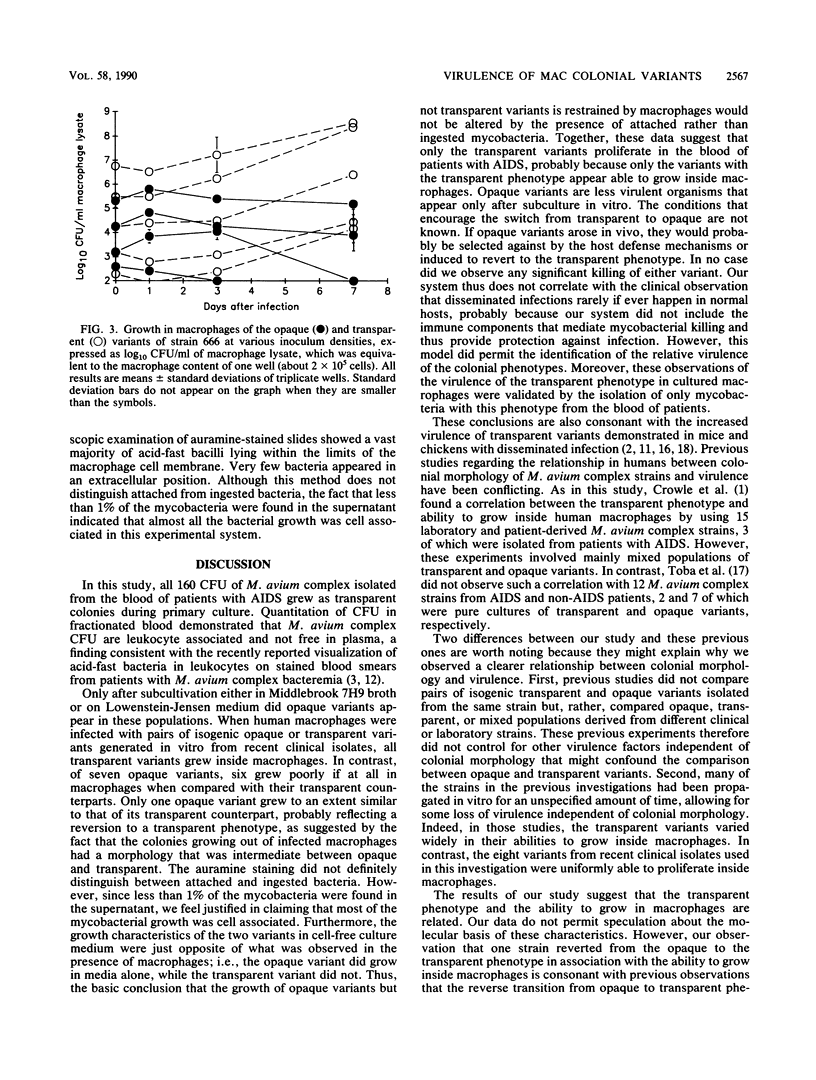
Abstract
Strains of the Mycobacterium avium complex (MAC) yield opaque and transparent colonial variants when cultivated in vitro. The transparent variants are more virulent than the opaque for animals, but little is known about the respective roles of these colonial variants in humans. To assess which variant infects humans, various blood fractions from eight patients with MAC bacteremia were plated directly onto 7H10 agar. In cell fractionation studies, all the M. avium complex CFU were associated with leukocytes and none were found free in plasma. All colonies on the primary culture plate exhibited the transparent phenotype. However, during subculture in 7H9 broth or on Lowenstein-Jensen agar, opaque variants appeared in seven of eight strains. Isogenic pairs of transparent and opaque variants were prepared and used to infect in vitro human monocyte-derived macrophages from healthy seronegative individuals. Transparent variants invariably grew inside macrophages, but only one of seven opaque variants did so. These observations indicate that the bacteremia of M. avium complex in acquired immunodeficiency syndrome patients consists exclusively of the transparent variants, perhaps because these variants are able to multiply inside macrophages. In contrast, opaque variants appear after in vitro subculture and are controlled by human macrophages, consistent with their reduced virulence in animals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar F. P., Pejovic I., Cacciatore R., Peric-Golia L., Runyon E. H. Mycobacterium intracellulare. Maintenance of pathogenicity in relationship to lyophilization and colony form. Scand J Respir Dis. 1968;49(2):153–162. [PubMed] [Google Scholar]
- Eng R. H., Bishburg E., Smith S. M., Mangia A. Diagnosis of Mycobacterium bacteremia in patients with acquired immunodeficiency syndrome by direct examination of blood films. J Clin Microbiol. 1989 Apr;27(4):768–769. doi: 10.1128/jcm.27.4.768-769.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Crawford J. T., Bates J. H. Association of plasmids and virulence of Mycobacterium avium complex. Am Rev Respir Dis. 1988 Jan;137(1):212–214. doi: 10.1164/ajrccm/137.1.212. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y., Fukunaga M., Taniguchi H. Plasmid deoxyribonucleic acid and translucent-to-opaque variation in Mycobacterium intracellulare 103. J Bacteriol. 1981 May;146(2):656–659. doi: 10.1128/jb.146.2.656-659.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Solotorovsky M. R. Relationship of colonial morphology to virulence for chickens of Mycobacterium avium and the nonphotochromogens. Am Rev Respir Dis. 1965 Nov;92(5):704–713. doi: 10.1164/arrd.1965.92.5.704. [DOI] [PubMed] [Google Scholar]
- Moffie B. G., Krulder J. W., de Knijff J. C. Direct visualization of mycobacteria in blood culture. N Engl J Med. 1989 Jan 5;320(1):61–62. doi: 10.1056/nejm198901053200115. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Susceptibilities of transparent, opaque, and rough colonial variants of Mycobacterium avium complex to various fatty acids. Antimicrob Agents Chemother. 1988 Mar;32(3):400–402. doi: 10.1128/aac.32.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Toba H., Crawford J. T., Ellner J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989 Jan;57(1):239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley C. L., David H. L. Effect of temperature on the rate of the transparent to opaque colony type transition in Mycobacterium avium. Antimicrob Agents Chemother. 1976 Jan;9(1):113–119. doi: 10.1128/aac.9.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]