Abstract
Background
Culture-independent methods based on the 16S ribosomal RNA molecule are nowadays widely used for assessment of the composition of the intestinal microbiota, in relation to host health or probiotic efficacy. Because Bifidobacterium thermophilum was only recently isolated from human faeces until now, no specific real-time PCR (qPCR) assay has been developed for detection of this species as component of the bifidobacterial community of the human intestinal flora.
Results
Design of specific primers and probe was achieved based on comparison of 108 published bifidobacterial 16S rDNA sequences with the recently published sequence of the human faecal isolate B. thermophilum RBL67. Specificity of the primer was tested in silico by similarity search against the sequence database and confirmed experimentally by PCR amplification on 17 Bifidobacterium strains, representing 12 different species, and two Lactobacillus strains. The qPCR assay developed was linear for B. thermophilum RBL67 DNA quantities ranging from 0.02 ng/μl to 200 ng/μl and showed a detection limit of 105 cells per gram faeces. The application of this new qPCR assay allowed to detect the presence of B. thermophilum in one sample from a 6-month old breast-fed baby among 17 human faecal samples tested. Additionally, the specific qPCR primers in combination with selective plating experiments led to the isolation of F9K9, a faecal isolate from a 4-month old breast-fed baby. The 16S rDNA sequence of this isolate is 99.93% similar to that of B. thermophilum RBL67 and confirmed the applicability of the new qPCR assay in faecal samples.
Conclusion
A new B. thermophilum-specific qPCR assay was developed based on species-specific target nucleotides in the 16S rDNA. It can be used to further characterize the composition of the bifidobacterial community in the human gastrointestinal tract. Until recently, B. thermophilum was considered as a species of animal origin, but here we confirm with the application of this new PCR assay the presence of B. thermophilum strains in the human gut.
Background
Real-time quantitative polymerase chain reaction (qPCR) has recently emerged as promising tool for faecal microbiota monitoring in animal and human faeces [1-3] since culture-based methods are not suitable for quantification of certain microbial groups, species or strains in faeces [4]. Due to the role of bifidobacteria as probiotics much attention has been focused on the qPCR-based quantification of both the autochthonous bifidobacteria in faecal microbiota and on selected strains of bifidobacteria after consumption as probiotics [5-9]. Compared to fluorescence in situ hybridization (FISH), the most widely used method for culture independent quantification in faeces, qPCR is less developed in terms of the availability of specific probes [10]. On the other hand qPCR was shown to be about a 10 to 100 fold more sensitive than culture- and FISH-based enumeration techniques [11], as well as to be rapid, easy and more accurate for quantification of low levels of bacteria [12]. Several oligonucleotides were designed for the Bifidobacterium species found in the human intestinal tract, most of them based on the 16S rDNA sequence [11,13]. Other target sequences like the transaldolase encoding gene [5], heat-shock protein (HSP60) gene [14], intergenic spacer of the 16S-23S rRNA gene [15] are also being investigated for species-specific detection and quantification. Oligonucleotides targeting such sequences could also be used for developing qPCR primers.
Bifidobacterium thermophilum, being considered as an animal-associated commensal species, was never included in studies on the bifidobacterial composition of the human intestinal flora and to our knowledge, no oligonucleotide was designed for the development of B. thermophilum-specific PCR or qPCR assay until now. Recently, design of a pair of oligonucleotides for PCR amplification of a portion of the 16S rDNA of B. thermophilum was reported, but effective specificity of the assay was questioned [16]. Previously, we have isolated and characterized bifidobacteria with anti-Listeria activity from stool of newborns [17,18]. Strain RBL67 was identified as B. thermophilum using 16S rDNA sequence homology, comparative HSP60 sequence analysis, DNA-DNA genome hybridization and carbohydrate fermentation patterns [19]. This was the first demonstration of the presence of B. thermophilum in human faeces. In this study, we designed oligonucleotides specific for B. thermophilum that we used to develop PCR and qPCR assays to study the distribution of this species in human faecal samples. Finally, the qPCR technology let us isolate a strain of B. thermophilum from human infant faeces closely related to B. thermophilum RBL67.
Results
Design of a B. thermophilum specific PCR assay
Specificity of the B. thermophilum specific primer was assessed by PCR on colonies with primers btherm and the Bifidobacterium genus-specific primer lm3 (Table 1). Of the 17 Bifidobacterium and two Lactobacillus strains tested, positive signals (amplification of a fragment of approximately 1.5 kb) were only obtained with the three faecal isolates B. thermophilum RBL67, Bifidobacterium thermacidophilum subsp. porcinum RBL68 and RBL70 (Figure 2, lanes 1 to 3, respectively), B. thermophilum DSM20210T (Figure 2, lane 6), and B. thermacidophilum subsp. porcinum LMG21689T (Figure 2, lane 5).
Table 1.
Oligonucleotides used in this study
| Oligonucleotide | Sequence 5'-3' | Reference |
| btherm | GAT GTG CCG GGC TCC TGC ATG | This study |
| lm3 | CGG GTG CTI CCC ACT TTC ATG | [20] |
| lm26 | GAT TCT GGC TCA GGA TGA ACG | [20] |
| bak11w | AGT TTG ATC MTG GCT CAG | [21] |
| bak4 | AGG AGG TGA TCC ARC CGC A | [22] |
| bthermRTF | TTG CTT GCG GGT GAG AGT | This study |
| bthermRTR | CGC CAA CAA GCT GAT AGG AC | This study |
| bthermTqM | *FAM-ATG TGC CGG GCT CCT GCA T-*TAMRA | This study |
| 520F | CAG GAG TGC CAG CAG CCG CGG | [23] |
| 520R | ACC GCG GCT GCT GGC | [23] |
| 1100F | CAG GAG CAA CGA GCG CAA CCC | [23] |
| 1100R | AGG GTT GCG CTC GTT G | [23] |
*FAM (6-carboxyfluorescein): fluorescent reporter dye. TAMRA (6-carboxytetramethylrhodamine): quencher.
Figure 2.
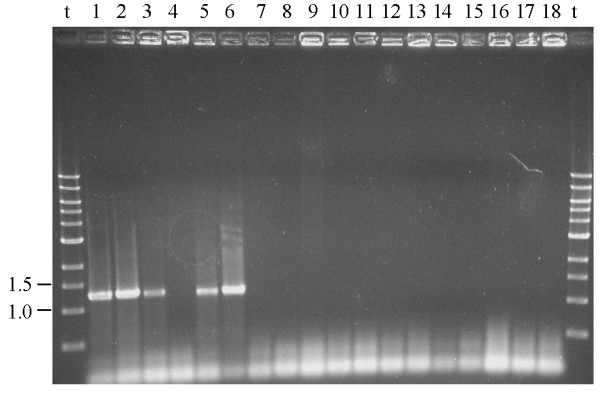
Specificity of the PCR for B. thermophilum using primers btherm and lm3. Agarose gel electrophoresis of PCR products (1.3 kb) obtained with primers btherm/lm3 on colonies of B. thermophilum RBL67 (1), B. thermacidophilum subsp. porcinum RBL68 (2), B. thermacidophilum subsp. porcinum RBL70 (3), B. thermacidophilum subsp. thermacidophilum LMG21395T (4), B. thermacidophilum subsp. porcinum LMG21689T (5), B. thermophilum DSM20210T (6), B. boum DSM20432T (7), B. breve DSM20213T (8), Bifidobacterium longum NCC2705 (9), Bifidobacterium coryneforme DSM2026T (10), Bifidobacterium asteroides DSM20089T (11), Bifidobacterium animalis subsp.lactis DSM10140 (12), B. animalis subsp. animalis DSM20105 (13), Bifidobacterium cuniculi DSM20435T (14), Bifidobacterium adolescentis DSM20083T (15), Bifidobacterium bifidum DSM20456T (16), Lactobacillus delbrueckii subsp. lactis DSM20072T (17) and Lactobacillus plantarum subsp.plantarum DSM20174T (18), t: Tridye 1-kb DNA ladder, in kb (New England Biolabs, Ipswich, MA, USA).
Detection of B. thermophilum in faecal DNA samples by PCR
Classical PCR analysis with the B. thermophilum specific primers btherm and lm3 (Table 1) on total DNA isolated from faecal samples spiked with known quantities of B. thermophilum RBL67 showed that the detection limit of the method was 108 B. thermophilum cells per gram faeces (Figure 3). This high detection limit did not allow DNA amplification from any of the 17 faecal samples. Efficacy of PCR amplification on faecal DNA samples was confirmed by amplification of a 1.3 kb-DNA fragment from each faecal DNA sample generated with the Bifidobacterium-genus specific primer-pair lm26/lm3 [20] (data not shown).
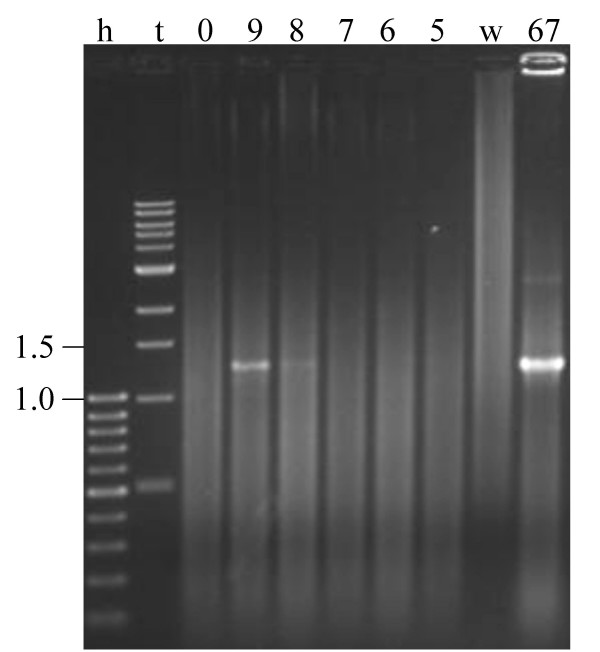
Figure 3.
Determination of the detection limit of the B. thermophilum PCR on faecal samples. PCR amplification with primers btherm and lm3 of DNA isolated from faecal sample F0, the PCR-amplification negative control (0) spiked with 109 (9), 108 (8), 107 (7), 106 (6) or 105 (5) B. thermophilum RBL67 cells/g faeces; h: 100-bp DNA ladder [kb]; t: 1-kb DNA ladder [kb]; w: water; 67: PCR on a colony of B. thermophilum RBL67.
Development of a qPCR assay for detection of B. thermophilum in human faeces
The qPCR technology was chosen as an alterative to the classical PCR for its higher sensitivity. B. thermophilum specificity of qPCR performed with primers bthermRTF, bthermRTR and the TaqMan probe bthermTqM (Table 1) was tested by amplification of DNA isolated from six different Bifidobacterium strains belonging to four closely related species. A positive signal was obtained for B. thermophilum RBL67 (CT = 17.3 ± 0.5), B. thermophilum DSM20210T (CT = 24.9 ± 0.3) and the closely related species B. thermacidophilum subsp. porcinum LMG21689T (CT = 16.3 ± 0.4), but not for B. thermacidophilum subsp. thermacidophilum LMG21395T, Bifidobacterium breve DSM20213T, and Bifidobacterium boum DSM20432T. Amplification of DNA from B. thermophilum RBL67 with this new assay was shown to be linear for DNA concentrations ranging from 0.02 ng/μl (CT = 28.1 ± 0.3) to 200 ng/μl (CT = 15.3 ± 0.4) with a regression coefficient R2 = 0.991 (data not shown).
Screening of faecal samples by qPCR
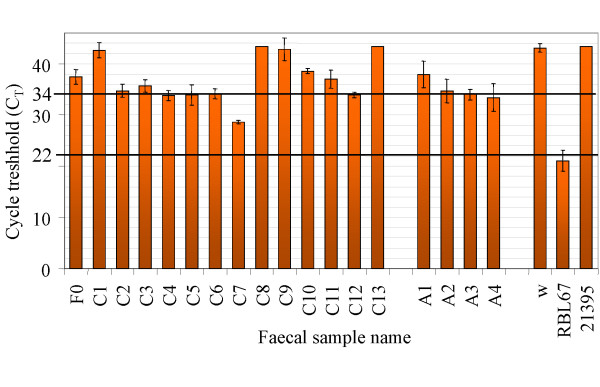
As for the classical PCR approach, the detection limit of the qPCR assay was determined by analysis of DNA isolated from spiked faecal samples and was equal to 1 × 105 bacterial cells per gram of faeces. Detection in faecal samples was shown to be linear between 1 × 109 and 1 × 105 cells per gram of faeces and corresponded to CT values comprised between 21.6 ± 0.6 and 33.8 ± 0.1, respectively, with a regression coefficient R2 = 0.995 (data not shown). One of the 17 faecal samples (sample C7 from a 6 month-old breast-fed baby) gave a positive signal within this range (Figure 4) with a CT value of 28.6 ± 0.3, corresponding to a concentration of 5 × 106 cells per gram faeces.
Figure 4.
Detection of B. thermophilum in faecal samples by qPCR targeting the 16S rDNA. Cycle threshold (CT) values measured for faecal samples from 13 children (C1 to C13) and 4 adults (A1 to A4). F0: PCR-negative faecal sample, w: water instead of DNA, RBL67: DNA from pure culture of RBL67, 21395: DNA from B. thermacidophilum subsp. thermacidophilum LMG21395T. Values are means and standard deviations for three repetitions of the qPCR assay with three replicates each.
Application of the newly developed qPCR assay for faecal samples
Raffinose-Bifidobacterium (RB) medium and MRSC-NNLP were tested for isolation of new B. thermophilum strains from human faeces. MRSC-NNLP was chosen for isolation of bifidobacteria because it allowed a better growth of RBL67 (1.5 × 109 cfu/ml after three days of incubation at 40°C, in comparison to 2.9 × 107 cfu/ml in RB-medium and under the same conditions) and was easier to prepare. Approximately 1–5 × 108 colonies per gram faeces could be cultivated from all of the 17 samples plated on MRSC-NNLP agar. Microscopic observations of the isolates showed that the medium was not completely selective, allowing for the growth of non-rod-shaped microorganisms. 60 rod-shaped microorganisms were selected for PCR analysis on colony. Twenty-five of them were positive with the Bifidobacterium genus specific primers lm26/lm3, but only one of them gave a positive signal with btherm/lm3. This isolate, F9K9 from a 4-month old breast-fed baby, was streaked several times on MRSC agar and the absence of contaminants other than Bifidobacterium was confirmed by three PCR reactions with lm26/lm3, btherm/lm3 and bak4/bak11w [24] (data not shown). Sequencing of the 16S rDNA fragment amplified with lm26 and lm3 yielded a 1454-bp sequence which was 99.93% identical to the 16S rDNA of B. thermophilum RBL67. Sequence identities with other Bifidobacterium strains are summarized in Table 2.
Table 2.
16S rDNA sequence identities of isolate F9K9 with published 16S rDNA sequences
| Accession | Description | % I* |
| DQ340557.1 | B. thermophilum RBL67 [19] | 99.93 |
| AY148470.1 | B. thermacidophilum subsp. porcinum LMG21689T [28] | 99.64 |
| D86190.1 | B. boum JCM1211 (DSM20432T) | 98.15 |
| AB016246.1 | B. thermacidophilum subsp.thermacidophilum LMG21395T [27] | 97.02 |
| U10151.1 | B. thermophilum ATCC25525T (DSM20210T) | 95.82 |
| D89330.1 | B. saeculare DSM6533 | 95.29 |
| AF491832 | B. breve JCM1273 (= DSM20091) | 95.16 |
*The percentage of identity (% I) was determined by comparison of the sequence of F9K9 against the sequences present in the database with the BLAST tool from NCBI.
Discussion
Real-time PCR (qPCR) is known to be a more sensitive technique than classical PCR. This is reflected by our results for specific amplification of 16S rDNA from spiked faecal samples, where changing from classical PCR to qPCR for the detection of B. thermophilum in faecal samples lowered the detection limit of the assay from 108 to 105 cells per gram faeces. The high sensitivity obtained for qPCR in this study is similar to detection limits reported by different groups for other Bifidobacterium species or genus specific qPCR assays. Matsuki et al. [11], Penders et al. [6] and Gueimonde et al. [25], for example, reported detection limits of 106, 5 × 103 and 5 × 104 cells of Bifidobacterium spp. per gram faeces, respectively. The application of a recent qPCR technology using rRNA as target molecule combined with reverse transcriptase could further enhance the sensitivity down to 103 cells/g faeces [26]. This methodology could also be developed for the detection of other subdominant faecal bacteria such as B. thermophilum.
We have developed a qPCR assay which is specific for B. thermophilum although the assay is also positive for the type strain of B. thermacidophilum subsp.porcinum, and B. thermacidophilum subsp.porcinum RBL68 and RBL70 (these subspecies were originally named "suis" which is a synonym for "porcinum"), but not with B. thermacidophilum subsp. thermacidophilum (LMG21395T) [27]. Based on our published data including DNA-DNA genome hybrizations [19] we underline that B. thermacidophilum subsp. porcinum [28] should belong to the B. thermophilum species and consequently, we conclude that our qPCR system is specific for B. thermophilum.
Until now, B. thermophilum was considered as an animal-associated species, mainly present in faeces of ruminants and pigs. The amplification of a specific 16S rDNA sequence with our qPCR on the children faecal sample C7 as well as the isolation of a B. thermophilum isolate from children faeces during this work support the assumption of von Ah et al. [19] that presence of B. thermophilum in food cannot be used to discriminate between animal and human bacterial contamination, as previously suggested [29].
Conclusion
This is the first report of the development of a qPCR assay for specific detection of B. thermophilum, a species that was not included in analysis of the composition of the bifidobacterial human intestinal microbiota until now. Using this assay, we detected B. thermophilum at a concentration of 5 × 106 cells per gram in one faeces sample, confirming the presence of this species in human faecal material.
Methods
Bacterial strains and culture conditions
Unless otherwise indicated, bifidobacteria and lactobacilli were grown in liquid cultures overnight in 10 ml MRSC medium consisting of MRS [30], obtained from Biolife (Milan, Italy) and supplemented with 0.05% L-cysteine hydrochloride, or on MRSC-agar plates (MRSC supplemented with 1.5% w/v agar). Incubation was carried out for 24 h at 37°C in anaerobic jars with an anaerobic atmosphere generation system (Oxoid AnaeroGen TM, Basel, Switzerland). B. thermophilum RBL67, as well as B. thermophilum subsp. porcinum RBL68 and RBL70 are human infant faecal isolates [17-19,31]. All of the other strains are commercial strains from DSMZ (German collection of microorganisms and cell cultures, Braunschweig, Germany) or LMG (Laboratories for Microbiology and Microbial Genetics, Ghent, Belgium).
Isolation of bifidobacteria from faecal samples
Seventeen faecal samples from human adults (4) and breast-fed children between 1 to 6 months (13) were collected as already described [32]. Subjects or parents of the subjects were informed orally and in writing about the aims and procedures of the study and consent was obtained from them. The study protocol was reviewed and approved by the Ethical Committees of the canton of Zurich and the SPUK-committee of the University Children's Hospital of Zurich (project StV31/05).
For efficient growth of B. thermophilum strains from faecal samples, Raffinose-Bifidobacterium (RB) [33] and MRSC-NNLP [34] media were compared. Serial 10-fold dilutions of overnight cultures of B. thermophilum RBL67 (containing approximately 109 cfu/ml) in saline solution (8.5 g/Liter NaCl, 1 g/Liter peptone, 0.05% cysteine-HCl, pH 6–7) were plated on RB and on MRSC-NNLP, incubated for 3 days anaerobically at 40°C and cell counts were determined. Incubation temperature of 40°C was chosen as an additional selective condition, due to the relative heat tolerance of B. thermophilum spp. [19]. For isolation of bifidobacteria from faecal samples, 20 mg of samples were homogenized by vigorous vortexing in 0.2 ml of saline solution, 10-fold serially diluted in the same solution and spread on MRSC-NNLP agar plates. Plates were incubated for 3 days under anaerobic conditions at 40°C and single isolates were observed under light microscope. Rod-shaped bacteria were selected for further analysis.
DNA purification methods
Total DNA was isolated from pure cultures of B. thermophilum RBL67, B. thermophilum DSM20210T, Bifidobacterium thermacidophilum subsp. porcinum LMG21689T, B. thermacidophilum subsp. thermacidophilum LMG21395T, Bifidobacterium breve DSM20213T and Bifidobacterium boum DSM20432T according to Leenhouts et al. [35]. Total DNA was prepared from 200 mg of 17 faecal samples using the QiAamp DNA Stool Mini kit (Qiagen, Basel, Switzerland) according to manufacturer's instructions. A PCR-amplification negative faecal sample (F0) was prepared by autoclaving twice one of the samples. For determination of the detection limit, 10-μl aliquots of F0 were spiked before DNA preparation with a 10-fold serial dilution of B. thermophilum RBL67 (overnight culture in MRSC) at concentrations ranging from 109 to 101 bacterial cells per g faeces. The extracted DNA was stored at -20°C.
DNA sequencing, PCR and qPCR reactions
Primers and probe used in this study were synthesized by Microsynth and are listed in Table 1. The TaqMan probe bthermTqM was labeled with 5'-FAM as fluorescent reporter dye and 3'-TAMRA as quencher. Classical PCR was performed either on 2 μl DNA prepared from faecal samples as described above, or on 40 μl cell suspensions. For that, one colony was picked from an agar plate and resuspended in 210 μl of sterile, double distilled water. A 50-μl classical PCR reaction consisted of 2.5 U EuroTaq-DNA-Polymerase (Digitana, Horgen, Switzerland), 1.5 mM magnesium chloride (Digitana), 0.2 mM dNTP's (GE Healthcare) and 0.5 μM of each primer. When DNA isolated from faecal samples was used as template, 0.1 μg/ml BSA was added to the PCR reaction. Amplification conditions were as follows: 3 min at 95°C, 40 cycles of 15 sec at 95°C, 30 sec at 62°C and 2 min at 72°C, followed by 7 min at 72°C. Sequencing of the PCR product for 16S rDNA was performed by Microsynth (Balgach, Switzerland) using the primers btherm, 520F, 520R, 1100F, 1100R and lm3 (Table 1).
The qPCR reactions were set in a total volume of 25 μl, containing 2.5 μl of DNA extracted from faecal samples with the stool kit as described above, 12.5 μl of qPCR MasterMix from Eurogentec (Seraing, Belgium), 0.3 μM of each primer and 0.1 μM of the TaqMan probe. Reactions were run on an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Rotkreuz, Switzerland). The amplification conditions were 2 min at 50°C, 10 min denaturation at 95°C, followed by 45 cycles of 15 sec at 95°C and 1 min at 60°C. The cycle threshold (CT), corresponding to the number of cycles after which the target-DNA concentration increase becomes exponential, was monitored. Results were analyzed using the SDS 2.1 Software (Applied Biosystems). All reactions were done in triplicate and repeated at least twice (three times when faecal DNA extract was used as a template). Values in the text are mean ± SD.
Design of a B. thermophilum specific primer and qPCR assay
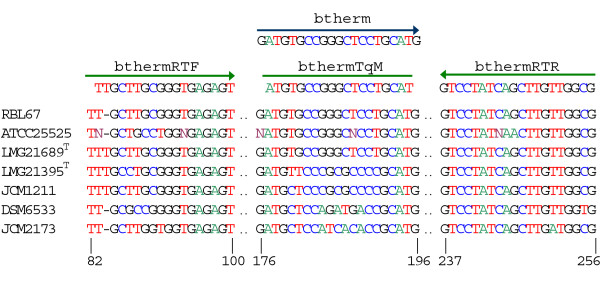
One hundred and eight sequences of bifidobacterial 16S rDNA of more than 1 kb in length were retrieved from the EMBL nucleotide sequence database http://www.ebi.ac.uk/embl and were used, together with the sequence of the 16S rDNA of B. thermophilum RBL67 [GenBank: DQ340557], to prepare a multiple alignment with ClustalW in the sequence analysis program BioEdit [36]. The primer btherm (Table 1) was manually designed in a variable region. Specificity of the primer was verified in silico with FASTA and the BLAST program from NCBI (version 2.2.15). For B. thermophilum-specific PCR amplification, primer btherm was used together with the Bifidobacterium-genus-specific reverse primer lm3. Cell suspensions of B. thermophilum RBL67 and DSM20210T, B. thermacidophilum strains RBL68, RBL70, LMG21395T and LMG21689T, B. boum DSM20432T, B. breve DSM20213T, B. longum DSM20219T, B. coryneforme DSM2026T, B. asteroides DSMZ20089T, B. animalis subsp. lactis DSM10140, B. animalis subsp. animalis DSM20105, B. cuniculi DSM20435T, B. adolescentis DSM20083T, B. bifidum DSM20456T, Lactobacillus delbrueckii subsp. lactis DSM20072T and Lactobacillus plantarum subsp. plantarum DSM20174T were used as template for amplification with btherm and lm3 under the conditions described below to test the specificity of the PCR. For the development of the qPCR assay, the btherm primer was modified to fit the lower melting temperature required for a TaqMan probe and adequate adjacent forward and reverse primers were designed with the program Primer3 [37] (Table 1). Aliquots of 5 μl of DNA (20 ng/μl) isolated from pure cultures of B. thermophilum RBL67, B. thermophilum DSM20210T, B. thermacidophilum subsp. porcinum LMG21689T, B. thermacidophilum subsp. thermacidophilum LMG21395T, B. breve DSM20213T and B. boum DSM20432T were amplified with this assay to assess its specificity. Localization of the primers btherm, bthermRTF and bthermRTR and of the TaqMan probe bthermTqM on an alignment of 16S rDNA sequences of seven bifidobacteria is shown in Figure 1.
Figure 1.
Localization of the 16S rDNA targets for oligonucleotides designed in this study. Multiple alignment of 16S rDNA sequences of B. thermophilum RBL67 [GenBank: DQ340557.1], B. thermophilum ATCC25525T [GenBank: U10151.1], B. thermacidophilum subsp. porcinum LMG21689T [GenBank: AY148470.1], B. thermacidophilum subsp.thermacidophilum LMG21395T [GenBank: AB016246.1], B. boum JCM1211 [GenBank: D86190.1], Bifidobacterium saeculare DSM6533 [GenBank: D89330.1] and B. breve JCM1273 [GenBank: AF491832]. Numbers correspond to E. coli 16S rDNA positions.
Authors' contributions
SM participated in the study conception and coordination, drafted the manuscript and designed the specific oligonucleotides. RM performed part of the experiments. LM and CL provided guidance during all parts of the work. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank Christian Braegger of the University Children's Hospital, Zurich for his helpful sampling coordination. This work was supported by the Swiss National Foundation (Project no. 3100A0-102256).
Contributor Information
Sophie Mathys, Email: sophie.mathys@ilw.agrl.ethz.ch.
Christophe Lacroix, Email: christophe.lacroix@ilw.agrl.ethz.ch.
Raffaella Mini, Email: minir@student.ethz.ch.
Leo Meile, Email: leo.meile@ilw.agrl.ethz.ch.
References
- Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, Tanaka R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl Environ Microbiol. 2004;70:167–173. doi: 10.1128/AEM.70.1.167-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT, Furrie E, Fite A, Macfarlane S. Characterisation of intestinal bacteria in infant stools using real-time PCR and Northern hybridisation analyses. FEMS Microbiol Ecol. 2005;54:77–85. doi: 10.1016/j.femsec.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kawaji S, Taylor DL, Mori Y, Whittington RJ. Detection of Mycobacterium avium supsp. paratuberculosis in ovine faeces by direct quantitative PCR has similar or greater sensitivity compared to radiometric culture. Vet Microbiol. 2007;125:36–48. doi: 10.1016/j.vetmic.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Blaut M, Collins MD, Welling GW, Doré J, van Loo J, de Vos W. Molecular biological methods for studying the gut microbiota: the EU human gut flora project. Br J Nutr. 2002;87:S203–S211. doi: 10.1079/BJN/2002539. [DOI] [PubMed] [Google Scholar]
- Requena T, Burton J, Matsuki T, Munro K, Simon MA, Tanaka R, Watanabe K, Tannock GW. Identification, detection, and enumeration of human Bifidobacterium species by PCR targeting the transaldolase gene. Appl Environ Microbiol. 2002;68:2420–2427. doi: 10.1128/AEM.68.5.2420-2427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243:141–147. doi: 10.1016/j.femsle.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Maruo T, Sakamoto M, Toda T, Benno Y. Monitoring the cell number of Lactococcus lactis subsp. cremoris FC in human faeces by real-time PCR with strain-specific primers designed using the RAPD technique. Int J Food Microbiol. 2006;110:69–76. doi: 10.1016/j.ijfoodmicro.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Fuijimoto J, Matsuki T, Sasamoto M, Tomii Y, Watanabe K. Identification and quantification of Lactobacillus casei strain Shirota in human feces with strain-specific primers derived from randomly amplified polymorphic DNA. Int J Food Microbiol. 2008;126:210–215. doi: 10.1016/j.ijfoodmicro.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Delroisse JM, Boulvin AL, Permentier I, Dauphin RD, et al. Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res doi: 10.1016/j.micres.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Vaughan EE, Heilig HG, Ben-Amor K, de Vos WM. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiol Rev. 2005;29:477–490. doi: 10.1016/j.femsre.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Watanabe K, Tanaka R. Genus- and species-specific PCR primers for the detection and identification of bifidobacteria. Curr Issues Intest Microbiol. 2003;4:61–69. [PubMed] [Google Scholar]
- Gueimonde M, Debor L, Tolkko S, Jokisalo E, Salminen S. Quantitative assessment of faecal bifidobacterial populations by real-time PCR using lanthanide probes. J Appl Microbiol. 2007;102:1116–1122. doi: 10.1111/j.1365-2672.2006.03145.x. [DOI] [PubMed] [Google Scholar]
- Satokari RM, Vaughan EE, Smidt H, Saarela M, Matto J, de Vos WM. Molecular approaches for the detection and identification of bifidobacteria and lactobacilli in the human gastrointestinal tract. Syst Appl Microbiol. 2003;26:572–584. doi: 10.1078/072320203770865882. [DOI] [PubMed] [Google Scholar]
- Delcenserie V, Bechoux N, China B, Daube G, Gavini F. A PCR method for detection of bifidobacteria in raw milk and raw milk cheese: comparison with culture-based methods. J Microbiol Methods. 2005;61:55–67. doi: 10.1016/j.mimet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Haarman M, Knol J. Quantitative real-time PCR assays to identify and quantify fecal Bifidobacterium species in infants receiving a prebiotic infant formula. Appl Environ Microbiol. 2005;71:2318–2324. doi: 10.1128/AEM.71.5.2318-2324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn SY, Seo JM, Ji GE. Evaluation of the PCR method for identification of Bifidobacterium species. Lett Appl Microbiol. 2008;46:7–13. doi: 10.1111/j.1472-765X.2007.02263.x. [DOI] [PubMed] [Google Scholar]
- Touré R, Kheadr E, Lacroix C, Moroni O, Fliss I. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J Appl Microbiol. 2003;95:1058–1069. doi: 10.1046/j.1365-2672.2003.02085.x. [DOI] [PubMed] [Google Scholar]
- Kheadr E, Dabour N, von Ah U, Lacroix C, Meile L, Fliss I. Genetic and phenotypic diversity of Bifidobacterium thermacidophilum fecal isolates from newborns. Can J Microbiol. 2007;53:1348–1359. doi: 10.1139/W07-101. [DOI] [PubMed] [Google Scholar]
- von Ah U, Mozzetti V, Lacroix C, Kheadr EE, Fliss I, Meile L. Classification of a moderately oxygen-tolerant isolate from baby faeces as Bifidobacterium thermophilum. BMC Microbiol. 2007;7:79. doi: 10.1186/1471-2180-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P, Pfefferkorn A, Teuber M, Meile L. Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl Environ Microbiol. 1997;63:1268–1273. doi: 10.1128/aem.63.4.1268-1273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberger D, Perschil I, Ritzler M, Altwegg M. A simple "universal" DNA extraction procedure using SDS and proteinase K is compatible with direct PCR amplification. PCR Methods Appl. 1995;4:368–370. doi: 10.1101/gr.4.6.368. [DOI] [PubMed] [Google Scholar]
- Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rDNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32:335–351. doi: 10.1128/jcm.32.2.335-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Watanabe K, Watanabe T, Oyaizu H. Phylogenetic analysis of the genus Bifidobacterium and related genera based on 16S rDNA sequences. Microbiol Immunol. 1998;42:661–667. doi: 10.1111/j.1348-0421.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Schürch C. PhD thesis Nr 14676. Swiss Federal Institute of Technology, Dept of Agricultural and Food Sciences; 2002. Development of a novel DNA transformation system for bifidobacteria. [Google Scholar]
- Gueimonde M, Tolkko S, Korpimaki T, Salminen S. New real-time quantitative PCR procedure for quantification of bifidobacteria in human fecal samples. Appl Environ Microbiol. 2004;70:4165–4169. doi: 10.1128/AEM.70.7.4165-4169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Tsuji H, Takashi A, Kado Y, Nomoto K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl Environ Microbiol. 2007;73:32–39. doi: 10.1128/AEM.01224-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Xin Y, Jian W, Liu X, Ling D. Bifidobacterium thermacidophilum sp. nov., isolated from an anaerobic digester. Int J Syst Evol Microbiol. 2000;50:119–125. doi: 10.1099/00207713-50-1-119. [DOI] [PubMed] [Google Scholar]
- Zhu L, Li W, Dong X. Species identification of genus Bifidobacterium based on partial HSP60 gene sequences and proposal of Bifidobacterium thermacidophilum subsp. porcinum subsp. nov. Int J Syst Evol Microbiol. 2003;53:1619–1623. doi: 10.1099/ijs.0.02617-0. [DOI] [PubMed] [Google Scholar]
- Delcenserie V, Bechoux N, Leonard T, China B, Daube G. Discrimination between Bifidobacterium species from human and animal origin by PCR-restriction fragment length polymorphism. J Food Prot. 2004;67:1284–1288. doi: 10.4315/0362-028x-67.6.1284. [DOI] [PubMed] [Google Scholar]
- de Man JD, Rogosa M, Sharpe ME. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- Moroni O, Kheadr E, Boutin Y, Lacroix C, Fliss I. Inactivation of adhesion and invasion of food-borne Listeria monocytogenes by bacteriocin-producing Bifidobacterium strains of human origin. Appl Environ Microbiol. 2006;72:6894–6901. doi: 10.1128/AEM.00928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys S, von Ah U, Lacroix C, Staub E, Mini R, Cereghetti T, Meile L. Detection of the pediocin gene pedA in strains from human faeces by real-time PCR and characterization of Pediococcus acidilactici UVA1. BMC Biotechnol. 2007;7:55. doi: 10.1186/1472-6750-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartemink R, Kok BJ, Weenk GH, Rombouts FM. Raffinose-Bifidobacterium (RB) agar, a new selective medium for bifidobacteria. J Microbiol Methods. 1996;27:33–43. doi: 10.1016/0167-7012(96)00926-8. [DOI] [Google Scholar]
- Dave RI, Shah NP. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus, and bifidobacteria. J Dairy Sci. 1996;79:1529–1536. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- Leenhouts KJ, Kok J, Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989;55:394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editor. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Press H. Totowa; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]