Abstract
The glyoxylate cycle is regarded as essential for postgerminative growth and seedling establishment in oilseed plants. We have identified two allelic Arabidopsis mutants, icl-1 and icl-2, which lack the glyoxylate cycle because of the absence of the key enzyme isocitrate lyase. These mutants demonstrate that the glyoxylate cycle is not essential for germination. Furthermore, photosynthesis can compensate for the absence of the glyoxylate cycle during postgerminative growth, and only when light intensity or day length is decreased does seedling establishment become compromised. The provision of exogenous sugars can overcome this growth deficiency. The icl mutants also demonstrate that the glyoxylate cycle is important for seedling survival and recovery after prolonged dark conditions that approximate growth in nature. Surprisingly, despite their inability to catalyze the net conversion of acetate to carbohydrate, mutant seedlings are able to break down storage lipids. Results suggest that lipids can be used as a source of carbon for respiration in germinating oilseeds and that products of fatty acid catabolism can pass from the peroxisome to the mitochondrion independently of the glyoxylate cycle. However, an additional anaplerotic source of carbon is required for lipid breakdown and seedling establishment. This source can be provided by the glyoxylate cycle or, in its absence, by exogenous sucrose or photosynthesis.
The aim of this study was to investigate the role of the glyoxylate cycle in the postgerminative growth of the oilseed Arabidopsis thaliana. Seedling establishment is one of the most critical phases in the life cycle of a higher plant. To achieve photoautotrophism, seedlings must adapt both developmental and metabolic programs to the prevailing environmental conditions (1). Seed storage reserves fuel this process. In oilseeds, a massive conversion of triacylglycerol to sugar occurs after germination (2). β-Oxidation of fatty acids derived from triacylglycerol produces acetyl-CoA, which ultimately is converted to sucrose through the glyoxylate cycle and gluconeogenesis. The sucrose produced is transported throughout the seedling, where it supports growth and development (2–4).
The glyoxylate cycle is a modified form of the tricarboxylic acid cycle that was initially discovered in microorganisms (5). This pathway plays a fundamental role in nature by allowing a variety of organisms to grow on 2-carbon compounds such as acetate or ethanol (5). Two enzymes unique to the glyoxylate cycle, namely malate synthase (MS) and isocitrate lyase (ICL), allow the decarboxylation steps of the tricarboxylic acid cycle to be bypassed, resulting in the net conversion of two molecules of acetyl-CoA into one of succinate. This pathway ultimately provides substrates for biosynthetic processes and respiration (5).
In addition to its established role in the postgerminative growth of oilseeds, the glyoxylate cycle has also been reported to operate as a salvage pathway in senescing plant tissues (6) and to have an anaplerotic role in carbohydrate-starved tissue (7), similar to the role that was described in microorganisms (5).
Work carried out by van Roermund and coworkers (8, 9) has demonstrated that the glyoxylate cycle is not required for growth of Saccharomyces cerevisiae on fatty acids. S. cerevisiae utilizes an alternative pathway for the metabolism of acetyl units, derived from the β-oxidation of fatty acids. Acetyl units can be transported from the peroxisome to the mitochondrion in the form of acetylcarnitine, and the carbon can be subsequently used for respiration (9). Such a mechanism has not been reported in plants. Furthermore, plant lipids are thought not to be a quantitatively important respiratory substrate even during the period of massive lipid mobilization that occurs in germinating oilseeds (10).
We are interested in determining the biochemical and genetic mechanisms that regulate oilseed germination and seedling establishment. Recent work on a mutant deficient in 3-ketoacyl-CoA thiolase, PED1, has demonstrated that peroxisomal β-oxidation is essential for postgerminative growth in Arabidopsis (11). The aim of our work was to establish whether the glyoxylate cycle is equally important or whether an alternative mechanism exists that allows utilization of fatty acids in plants.
Materials and Methods
Plant Material and Growth Conditions.
Seeds of Arabidopsis (Col0) were sterilized in 30% (vol/vol) sodium hypochlorite/0.001% Triton X-100 for 5 min and washed repeatedly with sterile water. They were then germinated on 0.8% (wt/vol) agar plates containing half-strength MS medium (12) at 22°C after 4 days imbibition at 4°C in the dark. Where stated, 1% (wt/vol) sucrose was included in the media. Seedling establishment was scored 4 weeks after germination as the ability to develop true foliage leaves.
Reverse Genetic Screening.
Genomic DNA pools from an En/Spm transposon-tagged Arabidopsis (ecotype Col0) mutant population (13), consisting of ≈48,000 En-1 insertions, were screened by PCR for a knockout in ICL (GenBank accession no. AB025634). The gene-specific primers ICL51 (5′-GCAGAGGGAGGCAAGAATGAGCATG) and ICL31 (5′-TAACACTCGGCCTTGCTCATTTGAC) were used in combination with En-1 border primers En8130 and En250 (see ref. 13). Two mutant alleles (icl-1 and icl-2) were identified, and the insertion sites were mapped by sequencing PCR products spanning the borders. The recessive short hypocotyl phenotype of the icl mutant (see Fig. 2C) was used to isolate stable alleles from their progeny. Excision events leading to reversion of the phenotype and restoration of the ICL ORF were also identified (14).
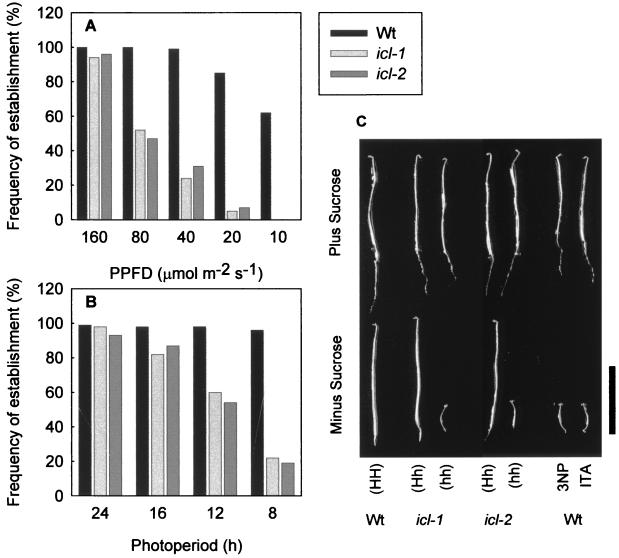
Figure 2.
The effect of light on icl mutant germination and seedling growth. (A and B) The effect of light intensity (A) and photoperiod (B) on the frequency of establishment of wild-type and icl mutant seedlings (as defined by the percentage of seedlings that develop true foliage leaves within 4 weeks after germination). PPFD, photosynthetic photon flux density. Two hundred seeds were grown under continuous light at various intensities or at 160 μmol⋅m−2⋅s−1 for various day lengths. (C) Five-day-old etiolated wild-type (Wt) and icl mutant seedlings that were grown on media in the presence and absence of 1% (wt/vol) sucrose. Wild-type seedlings also were grown on medium containing 0.1 mM 3-NP and 0.5 mM itaconate (ITA). (Scale bar = 1 cm.)
Northern Blotting.
Total RNA was extracted with the Purescript RNA isolation kit (Flowgen, Lichfield, U.K.) or with the hot phenol method (15). Total RNA (10 μg) was separated by electrophoresis using a 1.1% formaldehyde gel and was alkaline-blotted onto Zeta-probe membrane (Bio-Rad) by using 50 mM NaOH. Probes were prepared from ICL and MS cDNA clones (GenBank accession nos. T22831 and T04260, respectively), and membranes were hybridized by using the DIG (digoxigenin) system (Boehringer Mannheim) following the manufacturer's protocols.
Enzyme Assays.
Tissue extracts were prepared from Arabidopsis seedlings as described in ref. 16. Procedures for the enzyme assays were from the indicated references: acyl-CoA oxidase (17); ICL, MS, and 3-ketoacyl-CoA thiolase (18); and phosphoenolpyruvate carboxykinase (19). Protein content was determined according to the procedures in ref. 20, using BSA as a standard.
Metabolism of Acetate.
We incubated 100 2-day-old seedlings at 25°C in 0.2 ml of medium containing 1 mM sodium [1-14C]acetate (20 MBq⋅mmol−1) and 50 mM Mes/KOH (pH 5.2). Evolved 14CO2 was trapped in a well containing 50 μl of 5 M KOH. The reactions were stopped after 4 h and amounts of label incorporated into ethanol-soluble (acidic, neutral, and basic) components, ethanol-insoluble material, and CO2 were determined as described (3).
Lipid Analysis.
Fatty acids were extracted and measured by using the method previously described (21).
Results
Isolation of Mutants Deficient in ICL.
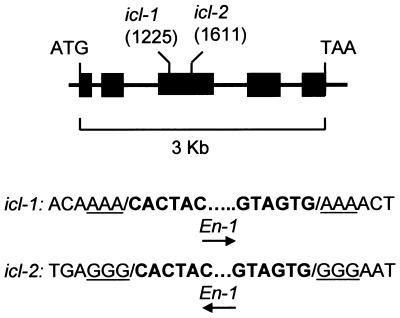
To establish the importance of the glyoxylate cycle to oilseed germination and seedling establishment, we have isolated Arabidopsis mutants that are disrupted in the ICL gene (GenBank accession no. AB025634). Only one ICL gene has been identified in Arabidopsis, and Southern blot analysis supports the presence of a single gene copy (data not shown). By using a PCR-based reverse-genetic screen, two independent mutants (icl-1 and icl-2) were isolated from an Arabidopsis population carrying the autonomous En/Spm transposable element from maize (13). The borders of the En-1 element are situated 1,225 bp 3′ of the start of translation in icl-1, whereas in icl-2 the element is in the reverse orientation, inserted at 1,611 bp. Both sites are within the third exon of the ICL gene (Fig. 1). By repeatedly selfing progeny of each mutant and by determining their phenotype, we identified individuals that showed no reversions in their offspring (sample size ≈2,000). One stable line was isolated for each mutant. Analysis of these lines revealed that they retained the termini of the En-1 element. They are therefore likely to be defective because of internal deletions (22). Germinal revertants also were isolated from both icl-1 and icl-2. In those revertants analyzed, the exact ICL ORF was restored (14). Phenotypic characterization was carried out on the stable mutant alleles.
Figure 1.
Identification of two icl mutants in Arabidopsis. Structure of the ICL gene showing the position of En-1 insertions in icl-1 and icl-2. Boxes and lines represent exons and introns, respectively. Nucleotide positions are relative to the translational start site. Nucleotides corresponding to the 3-bp target site duplication are underlined (14). The arrow indicates the orientation of the En-1 element.
Phenotypic Analysis.
Surprisingly, icl mutant seedlings grown in favorable conditions (160 μmol⋅m−2⋅s−1 photosynthetic photon flux density in continuous light at 22°C) showed no difference in the frequency of seed germination or seedling establishment as compared with the wild type (Fig. 2A). When light intensity was decreased or day length was shortened, germination frequency was not affected but the frequency of seedling establishment fell dramatically in the mutant (Fig. 2 A and B). Failure of seedlings to establish was defined by growth arrest ≈3 days after seed imbibition. Cotyledons expanded but failed to green, and the production of true foliage leaves was prevented. However, even under conditions of low light intensity or short days, a significant proportion of mutant seedlings could develop normally. Growth defects in icl mutant seedlings could be rescued by the provision of exogenous sugar (data not shown). After seedling establishment, no obvious vegetative or reproductive phenotype was observed in these mutants.
Hypocotyl elongation in dark-grown icl seedlings was strongly inhibited, and this phenotype could also be abolished by the provision of exogenous sugar (Fig. 2C). Analysis of heterozygotes revealed that the short-hypocotyl phenotype of dark-grown icl mutants is a recessive trait (Fig. 2C). Two chemical inhibitors of ICL enzyme activity, namely 3-nitropropionate (3-NP) and itaconate (23) could mimic the phenotype of the icl mutants when supplied exogenously to wild-type seedlings (Fig. 2C). Recently, Runquist and Kruger (23) have used 3-NP to demonstrate that ICL exerts a high level of control over gluconeogenesis in germinating castor bean endosperm (flux control coefficient ≈ 0.66). Our analysis reveals 3-NP is likely to be a specific inhibitor of ICL activity in vivo.
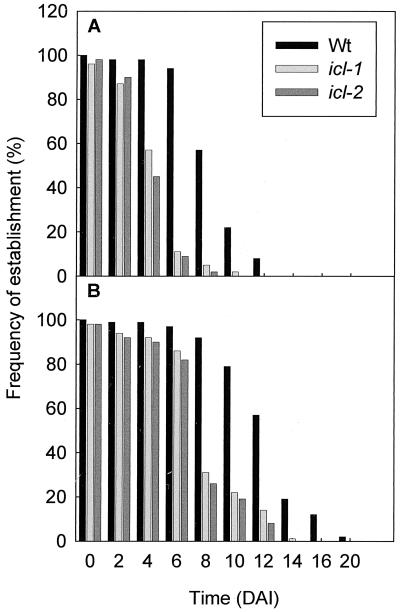
In soil, seedlings may be required to grow for several days in darkness before the cotyledons emerge into the light and photosynthesis can commence. To establish the importance of the glyoxylate cycle for the survival of etiolated seedlings, seeds were germinated and kept in the dark for increasing periods of time before being transferred to the light (Fig. 3A). The icl mutant shows a significant decrease in the frequency of seedling establishment upon transfer to continuous light after 4 days in the dark, and the effect is even more dramatic after 6 days. On the other hand, wild-type seedlings still show close to a 100% frequency of establishment after 6 days in the dark (Fig. 3A). The icl mutant seedlings are still alive after 6 days in the dark, because when they are transferred to medium supplemented with exogenous sucrose they can grow (Fig. 3B).
Figure 3.
The effect of periods of darkness, after germination, on the frequency of establishment of wild-type and icl mutant seedlings. DAI, days after imbibition. Two hundred seeds were germinated and grown in the dark for various periods and then transferred to continuous light (160 μmol⋅m−2⋅s−1) onto media without (A) and with (B) 1% (wt/vol) sucrose.
Gene Expression and Enzyme Activities.
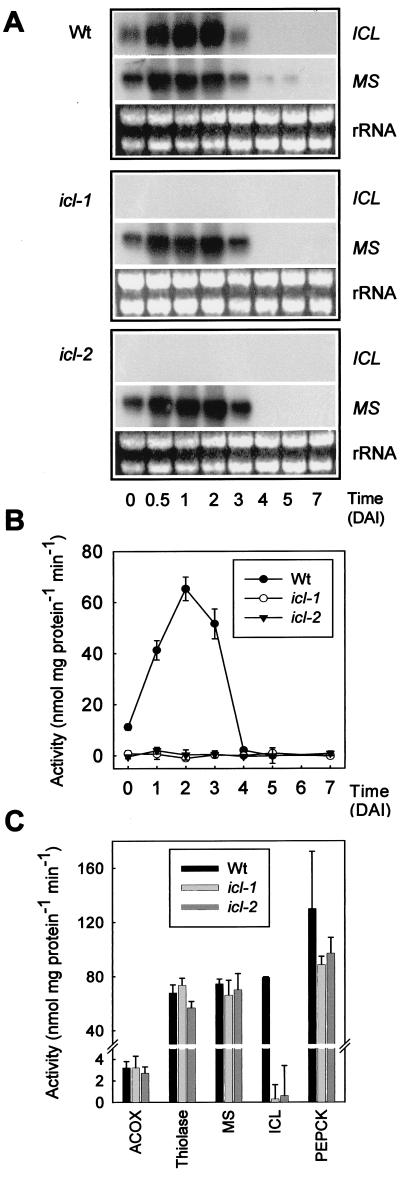
Northern analysis on RNA isolated from wild-type seeds and seedlings shows that both MS and ICL transcripts increase rapidly upon germination, remain high until day 3, and disappear at day 4 (Fig. 4A). This pattern is in agreement with previous reports (24–26). In contrast, the ICL transcript is undetectable in RNA isolated from icl mutant seedlings over the same period by using Northern analysis while MS transcript levels in the mutant are the same as those found in wild type (Fig. 4A). The lack of ICL transcript is mirrored by an absence of ICL enzyme activity in germinating seedlings (Fig. 4B). The activities of several other enzymes involved in fatty acid β-oxidation, the glyoxylate cycle, and gluconeogenesis are unaffected (Fig. 4C).
Figure 4.
Gene expression and enzyme activities in icl mutant seedlings. (A) Northern blots of ICL and MS transcripts in wild-type and icl mutant seedlings over the course of germination and seedling growth. DAI, days after imbibition. Total RNA (10 μg) was loaded in each lane, and rRNA is shown as a loading control. Seeds were germinated on medium containing sucrose in continuous light (80 μmol⋅m−2⋅s−1). (B) ICL activity in wild-type and icl mutant seedlings. (C) Activity of acyl-CoA oxidase (ACOX), measured by using palmitoyl-CoA as substrate; 3-ketoacyl-CoA thiolase, measured by using acetoacetyl-CoA as substrate; MS; ICL; and phosphoenolpyruvate carboxykinase (PEPCK) in 2-day-old wild-type and icl mutant seedlings. All activities are shown as mean ± SE of three replicates. Seeds were germinated on medium containing 1% (wt/vol) sucrose in continuous light (80 μmol⋅m−2⋅s−1).
Metabolism of Acetate.
Radiolabel feeding experiments show that in 2-day-old icl mutant seedlings, the proportion of 14C from [1-14C]acetate that is metabolized to sugars (measured as ethanol-soluble neutral material) is reduced by between 80% and 90% as compared with the wild type (Table 1). This finding confirms that the glyoxylate cycle is essential for the net conversion of acetate to sugar. The overall metabolism of exogenous acetate in the mutant also is decreased to almost half that of the wild type (Table 1). Respiration of acetate by the icl mutant is increased when exogenous sucrose is supplied in addition to acetate (data not shown).
Table 1.
Metabolism of [1-14C]acetate by 2-day-old icl mutant seedlings
| Seedling | Components, % of total
|
Total, 103 dpm | |||||
|---|---|---|---|---|---|---|---|
| Sugars | Amino acids | Organic acids | CO2 | Ethanol-insoluble | Other | ||
| Wild type | 23.3 ± 2.1 | 16.4 ± 3.1 | 13.4 ± 0.6 | 36.4 ± 4.5 | 4.6 ± 0.1 | 5.7 ± 0.2 | 105.2 ± 11.3 |
| icl-1 | 2.8 ± 0.3 | 20.5 ± 3.2 | 28.4 ± 0.9 | 40.3 ± 6.0 | 4.9 ± 0.1 | 3.3 ± 0.4 | 61.1 ± 4.8 |
| icl-2 | 4.0 ± 0.2 | 21.4 ± 1.9 | 25.9 ± 4.0 | 40.5 ± 3.7 | 3.0 ± 0.2 | 5.2 ± 0.2 | 57.5 ± 3.1 |
Seedlings were incubated for 4 h, and the incorporation of 14C into various components was determined. Values are presented as mean ± SE of four separate replicates. Seedlings were grown in continuous light (80 μmol⋅m−2⋅s−1).
Lipid Breakdown.
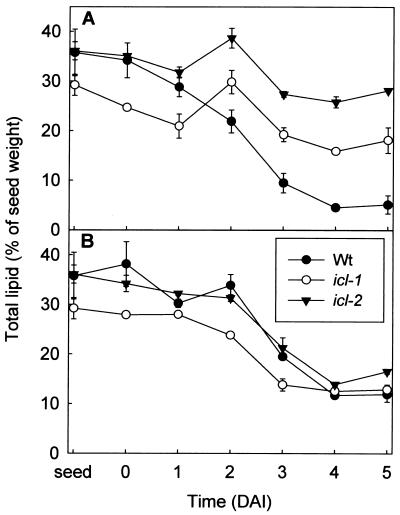
Disruption of the glyoxylate cycle did not alter the total fatty acid content or composition of mature icl seeds (data not shown). Analysis of total lipid content in dark-grown mutant seedlings revealed that fatty acid breakdown is severely inhibited when the seedlings are grown in the absence of exogenous sugars (Fig. 5A). Under these conditions, growth is arrested in the mutant. In the presence of exogenous sugars, the rate of breakdown is substantially increased and approximates that of wild-type seedlings (Fig. 5B). The decrease in eicosenoic acid in wild-type and mutant seedlings followed the same pattern as did that in total lipids (data not shown). This fatty acid is considered a marker of triacylglycerol in Arabidopsis (27). The presence of 1% (wt/vol) exogenous sucrose delays breakdown of lipid in wild-type seedlings (Fig. 5), suggesting that the breakdown process is subject to metabolic regulation.
Figure 5.
Total fatty acid content of etiolated wild-type and icl mutant seedlings grown on media in the presence (A) and absence (B) of 1% (wt/vol) sucrose. DAI, days after imbibition. Fatty acid content is expressed as a percentage of the seed dry weight. Values are shown as mean ± SE of measurements on three batches of 20 seedlings.
Discussion
This work shows that the glyoxylate cycle is not necessary for oilseed germination, nor is it required for seedling establishment when adequate light is available. The former observation is consistent with the argument that soluble carbohydrates that are present in many seeds are sufficient to support germination and that major storage reserves are used primarily during postgerminative growth (28). Lipid is the primary carbon store in Arabidopsis seeds, constituting between 30% and 40% of their dry weight. The fact that fatty acid breakdown is not required for Arabidopsis germination recently has been demonstrated by Hayashi and coworkers (11) with a mutant, ped1, deficient in the β-oxidation enzyme 3-ketoacyl-CoA thiolase. Analysis of the ped1 mutant also demonstrated that fatty acid breakdown is essential for seedling establishment, because ped1 seedling growth arrests shortly after germination (11).
Our data demonstrate that the glyoxylate cycle and therefore gluconeogenesis is not essential for Arabidopsis seedling establishment. This finding can be explained by the fact that fatty acid breakdown continues to occur in the mutant despite a block in the net conversion of acetate to carbohydrate. The acetyl units released from the β-oxidation of fatty acids in the icl mutants most probably are respired and thus support seedling establishment under optimal conditions. The physiological role and importance of β-oxidation is therefore different from that of the glyoxylate cycle during seedling establishment.
Lipids are not generally considered to be a quantitatively important respiratory substrate in plants (10). Surprisingly, our data reveal that in Arabidopsis seedlings, lipid can provide a major source of carbon for respiration. Seeds vary dramatically in their physiology (28). In the model oilseed castor (Ricinus communis), the major storage organ is the endosperm. This tissue is completely consumed during seedling development, and the oil within is converted almost stoichiometrically to sugars and then exported (4). In contrast, Arabidopsis seeds deposit storage reserves in the cotyledons of the embryo. After germination, seedling development is epigeal, and the cotyledons are progressively transformed into photosynthetic organs. Therefore, the glyoxylate cycle may be less predominant over respiration in Arabidopsis because there is a reduced need for the intercellular transport of carbon skeletons.
In S. cerevisiae, the pathway of β-oxidation also is required for growth on fatty acids, but the glyoxylate cycle is not essential (8, 9). Recently, these observations have been explained by the discovery of a carnitine/acetylcarnitine shuttle between the peroxisome and mitochondrion that allows carbon generated by β-oxidation to drive respiration (9). The existence of carnitine in plants and its involvement in lipid metabolism have remained unproven (29). Research using lettuce and sunflower seeds has indicated that fatty acids can be respired during germination, before the development of a fully functional glyoxylate cycle (30, 31). In these studies it is suggested that acetyl-CoA is converted to citrate within the peroxisome and that citrate is transferred to the mitochondrion. The movement of organic acids across the peroxisomal membrane has been demonstrated previously (32). In the absence of a mechanism for transporting acetyl-CoA, a similar process could be responsible for the movement of carbon skeletons derived from β-oxidation of fatty acids out of the peroxisome. The fact that plant cells do respire lipids also is implied by the fact that the capacity for β-oxidation is present in many tissues that lack the glyoxylate cycle (16, 33).
Our data indicate that the glyoxylate cycle plays an anaplerotic role in plants, supplying 4-carbon intermediates for the tricarboxylic acid cycle. This finding is suggested by the fact that icl mutant seedlings can break down fatty acids to provide carbon for respiration. However, lipid catabolism and growth are arrested if additional carbon is not supplied through glycolysis. This carbon can be provided either exogenously as sugars or in situ through the development of photosynthesis.
The glyoxylate cycle can be described as nonessential, because even under suboptimal growth conditions, which are prevalent in nature, a low percentage of icl mutant seed are still capable of seedling establishment. However, evolutionary conservation of the glyoxylate cycle is ensured, because disruption of the pathway reduces survivorship, placing null mutants at a competitive disadvantage. We used prolonged darkness to demonstrate that seedling survival and recovery upon transfer to light are severely compromised in the icl mutants as compared with wild type. These dark treatments, although extreme, effectively impose carbon-starvation conditions on the seedlings as storage reserves become exhausted in the wild type or cannot be utilized in the mutants because of the lack of the glyoxylate cycle. These results further demonstrate the importance of the glyoxylate cycle during Arabidopsis seedling development (7).
The glyoxylate cycle markers ICL and MS are expressed not only in germinating oilseeds but also in pollen (34), developing embryos (26), and senescing tissues (6). The mutants showed no obvious effect on seed set, seed quality, leaf senescence, or plant growth. More extensive analysis of these mutants, involving a range of different growth conditions, will enable researchers to address the physiological significance of the glyoxylate cycle in processes other than postgerminative growth.
In conclusion, this work shows that the glyoxylate cycle, unlike β-oxidation, is not essential for Arabidopsis seedling establishment. This difference can be explained by the fact that acetyl units, generated by the β-oxidation of fatty acids, can be metabolized by an alternative pathway to the glyoxylate cycle and most likely are respired. However, this alternative pathway cannot provide an anaplerotic supplement for the tricarboxylic acid cycle. Therefore, in the absence of the glyoxylate cycle, a further carbon source in addition to lipid is required for postgerminative growth. It will be interesting to establish whether plants use a similar mechanism to that described in S. cerevisiae for shuttling acetyl units between the peroxisome and mitochondrion or whether another mechanism operates.
Acknowledgments
We thank Ellen Wisman and Bernd Weisshaar for providing us with the opportunity to screen the En-1 mutagenized Arabidopsis population at the Max Planck Institute in Cologne, Germany. This work was funded by the Biotechnology and Biological Sciences Research Council through grants to S.M.S. and J.H.B. (RAS7677) and to I.A.G. (GAT9139).
Abbreviations
- MS
malate synthase
- ICL
isocitrate lyase
- 3-NP
3-nitropropionate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Holdsworth M, Kurup S, McKibbin R. Trends Plant Sci. 1999;4:275–280. [Google Scholar]
- 2.Kornberg H L, Beevers H. Nature (London) 1957;180:35–36. doi: 10.1038/180035a0. [DOI] [PubMed] [Google Scholar]
- 3.Canvin D T, Beevers H. J Biol Chem. 1961;236:988–995. [PubMed] [Google Scholar]
- 4.Beevers H. In: The Biochemistry of Plants. Stumpf P K, editor. Vol. 4. New York: Academic; 1980. pp. 117–130. [Google Scholar]
- 5.Kornberg H L, Krebs H A. Nature (London) 1957;179:988–991. doi: 10.1038/179988a0. [DOI] [PubMed] [Google Scholar]
- 6.Gut H, Matile P. Planta. 1988;176:548–550. doi: 10.1007/BF00397663. [DOI] [PubMed] [Google Scholar]
- 7.Graham I A, Denby K J, Leaver C J. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Roermund C W, Elgersma Y, Singh N, Wanders R J, Tabak H F. EMBO J. 1995;14:3480–3486. doi: 10.1002/j.1460-2075.1995.tb07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Roermund C W, Hettema E H, van Den Berg M, Tabak H F, Wanders R J. EMBO J. 1999;18:5843–5852. doi: 10.1093/emboj/18.21.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ap Rees T. In: The Biochemistry of Plants. Davies D D, editor. Vol. 2. New York: Academic; 1980. pp. 1–29. [Google Scholar]
- 11.Hayashi H, Toriyama K, Kondo M, Nishimura M. Plant Cell. 1998;12:183–195. doi: 10.1105/tpc.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murashige T, Skoog F. Physiol Plant. 1962;15:473–496. [Google Scholar]
- 13.Wisman E, Cardon G H, Fransz P, Saedler H. Plant Mol Biol. 1998;37:989–999. doi: 10.1023/a:1006082009151. [DOI] [PubMed] [Google Scholar]
- 14.Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, Saedler H, Weisshaar B. Proc Natl Acad Sci USA. 1998;95:12432–12437. doi: 10.1073/pnas.95.21.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay R, Chan A, Daly M, McPherson J. Science. 1987;236:1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- 16.Hooks M A, Kellas F, Graham I A. Plant J. 1999;19:1–13. doi: 10.1046/j.1365-313x.1999.00559.x. [DOI] [PubMed] [Google Scholar]
- 17.Hryb D J, Hogg J F. Biochem Biophys Res Commun. 1979;87:1200. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- 18.Cooper G, Beevers H. J Biol Chem. 1969;244:3507–3513. [PubMed] [Google Scholar]
- 19.Walker R P, Trevanion S J, Leegood R C. Planta. 1995;196:58–63. [Google Scholar]
- 20.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Browse J, Mc Court P J, Somerville C R. Anal Biochem. 1986;152:141–145. doi: 10.1016/0003-2697(86)90132-6. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz-Sommer Z, Gierl A, Cuypers H, Peterson P A, Saedler H. EMBO J. 1985;4:591–597. doi: 10.1002/j.1460-2075.1985.tb03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Runquist M, Kruger N J. Plant J. 1999;19:423–431. doi: 10.1046/j.1365-313x.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 24.Smith S M, Leaver C J. Plant Physiol. 1986;81:762–767. doi: 10.1104/pp.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen R D, Trelease R N, Thomas T L. Plant Physiol. 1988;86:527–532. doi: 10.1104/pp.86.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comai L, Deitrich R A, Maslyar D J, Baden C S, Harada J J. Plant Cell. 1989;1:293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemieux B, Miquel M, Somerville C, Browse J. Theor Appl Genet. 1990;80:234–240. doi: 10.1007/BF00224392. [DOI] [PubMed] [Google Scholar]
- 28.Bewley J D, Black M. Seeds: Physiology of Development and Germination. New York: Plenum; 1985. [Google Scholar]
- 29.Roughan G, Postbeittenmiller D, Ohlrogge J, Browse J. Plant Physiol. 1993;101:1157–1162. doi: 10.1104/pp.101.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salon C, Raymond P, Pradet A. J Biol Chem. 1988;263:12278–12287. [PubMed] [Google Scholar]
- 31.Ramond P, Spiteri A, Dieuaide M, Gerhardt B, Pradet A. Plant Physiol Biochem. 1992;30:153–161. [Google Scholar]
- 32.Mettler I J, Beevers H. Plant Physiol. 1980;66:555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooks M A, Fleming Y, Larson T R, Graham I A. Planta. 1998;207:385–392. doi: 10.1007/s004250050496. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J Z, Laudencia-Chingcuanco D L, Comai L, Li M, Harada J J. Plant Physiol. 1994;104:857–864. doi: 10.1104/pp.104.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]