Abstract
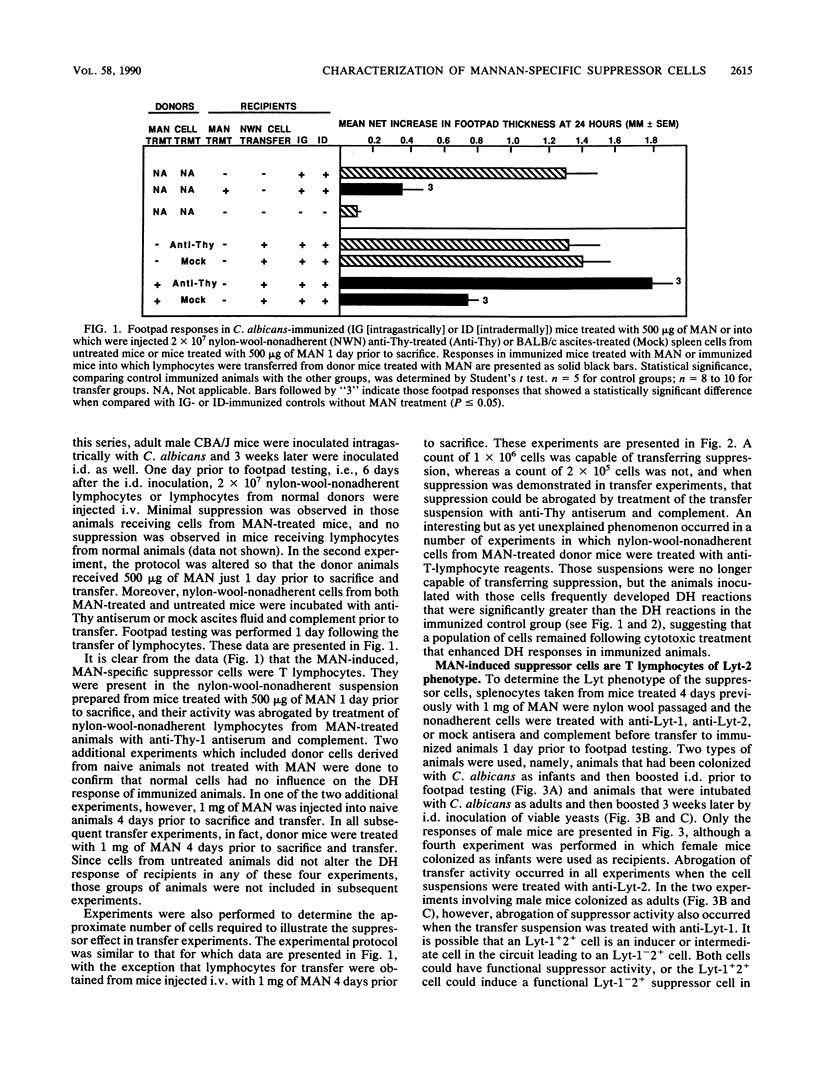
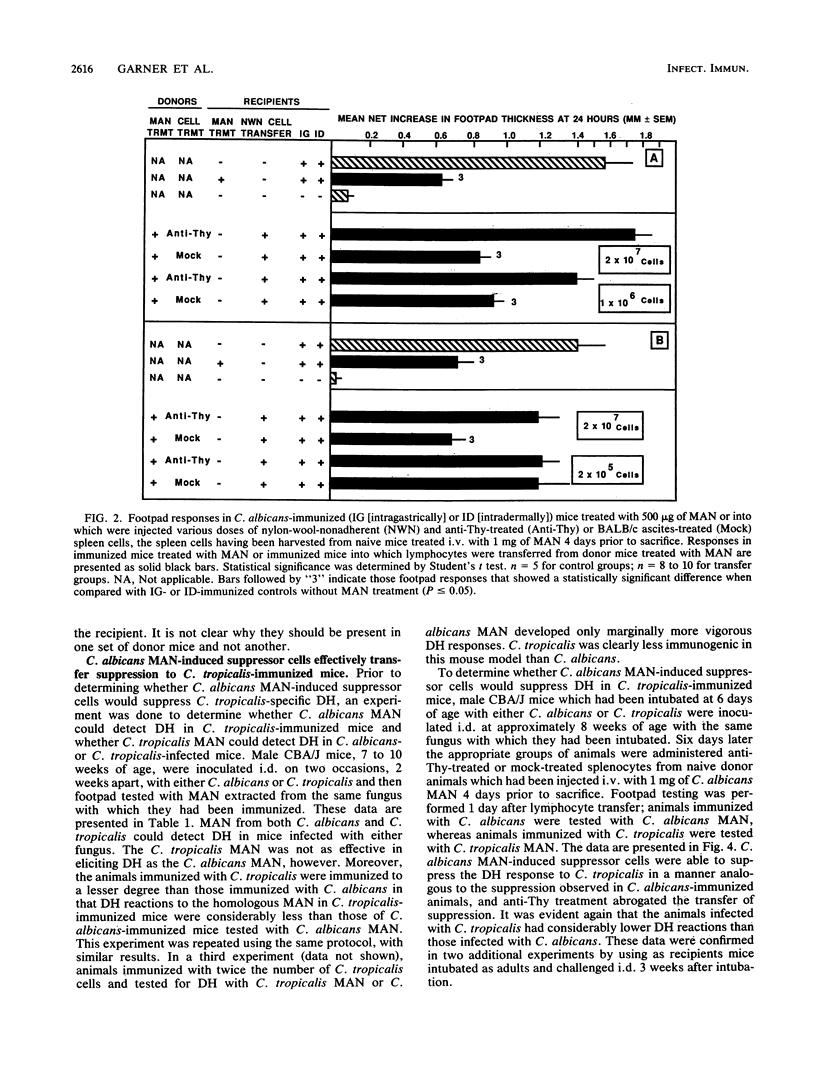
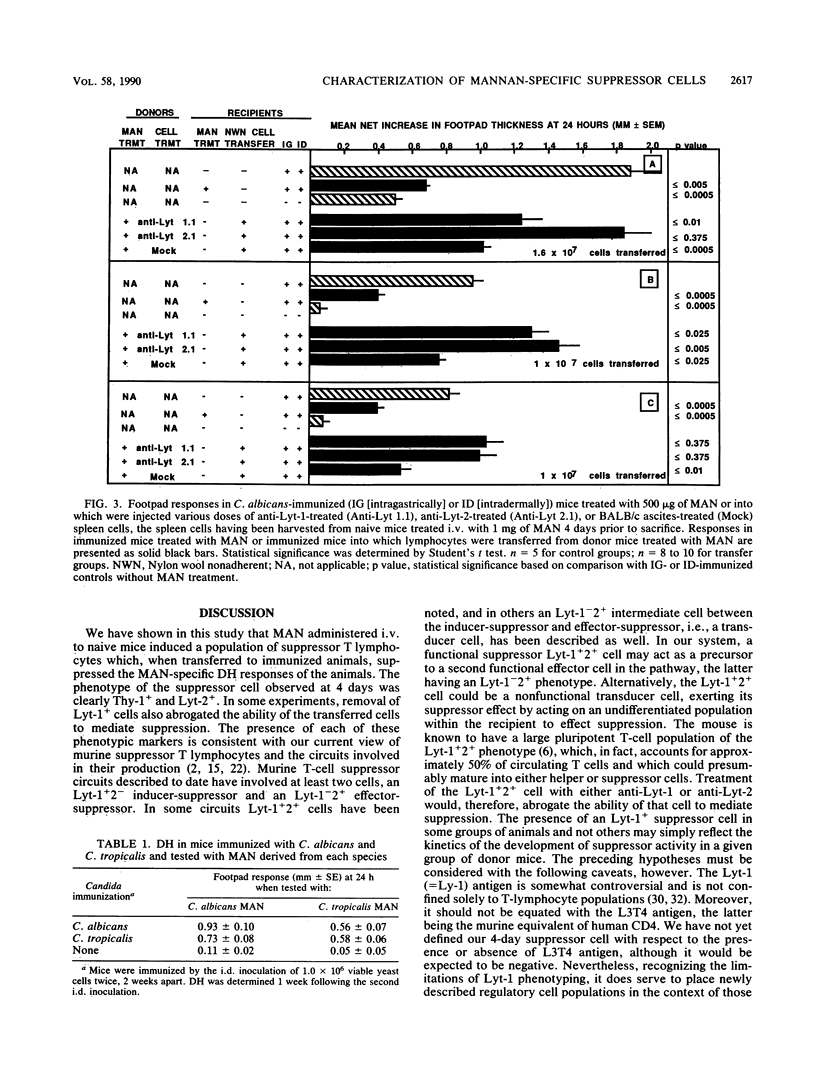
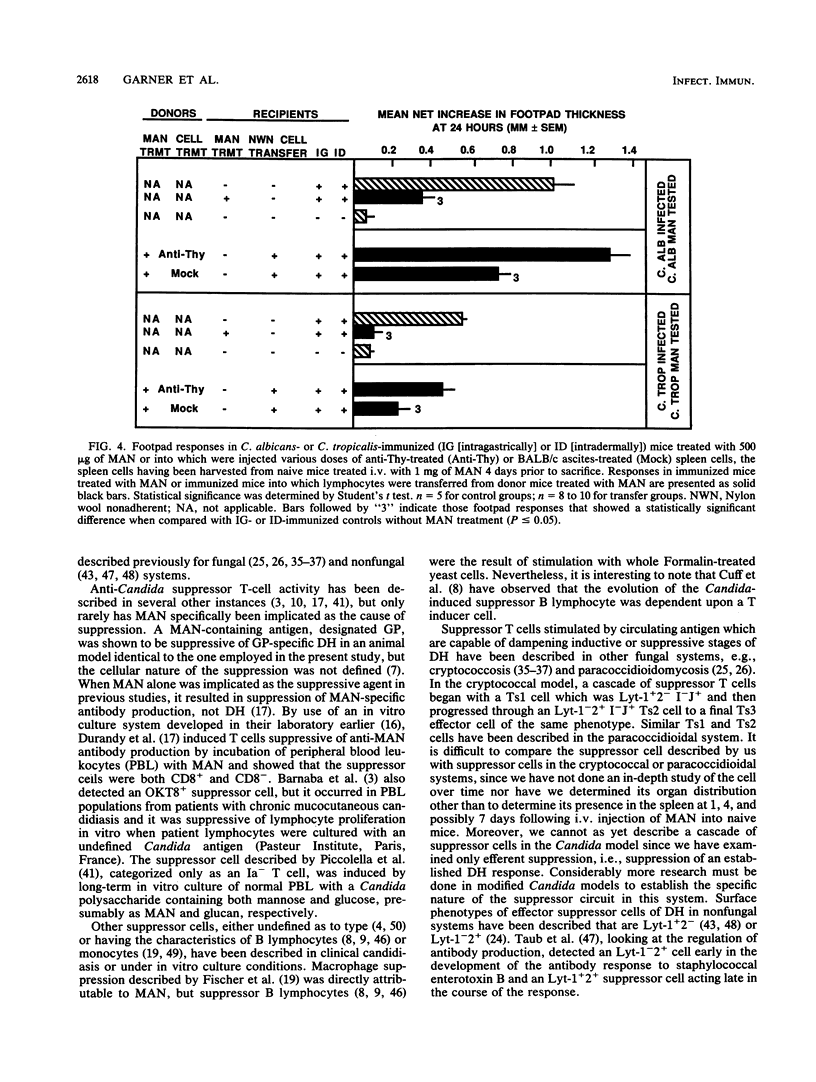
We have shown previously that CBA/J mice immunized with Candida albicans developed delayed hypersensitivity (DH) demonstrable with mannan (MAN) extracted from the same organism and that the intravenous (i.v.) injection of MAN prior to or during the immunization phase resulted in the suppression of the MAN-specific DH response. In this study, we demonstrate that MAN-induced suppression of DH is a T-lymphocyte-mediated phenomenon. Suppressor cells induced in vivo by the i.v. injection of MAN into naive mice 1 to 7 days prior to harvest were passaged through nylon wool, treated with various surface-specific antibodies and complement, and then injected i.v. into immunized syngeneic recipients. Enrichment of splenic T cells by passage over nylon wool and transfer of the nylon-wool-nonadherent populations to immunized recipient mice suppressed DH in a dose-dependent manner. Depletion of Thy+ or Lyt-2+ cells from nylon-wool-nonadherent populations regularly ablated the ability of such suspensions to transfer suppression. Treatment of the same transfer suspensions with anti-Lyt-1 had variable effects, suggesting that the surface density of the Lyt-1 antigen was not as constant from population to population as was the Lyt-2 antigen. In addition, C. albicans MAN-induced suppressor cells were able to suppress DH demonstrable with Candida tropicalis MAN in animals immunized with C. tropicalis. Suppression of DH by MAN in this model, therefore, is mediated by Thy+ Lyt-2+ lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson I. K., Rieger C. H., Soltani K., Tkalcevic V., Chan W. C., Lorincz A. L., Matz G. Late onset chronic mucocutaneous candidiasis with lymphoma and specific serum inhibitory factor. Cancer. 1979 Jan;43(1):101–108. doi: 10.1002/1097-0142(197901)43:1<101::aid-cncr2820430116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Colizzi V., Zembala M. An overview of T-suppressor cell circuits. Annu Rev Immunol. 1986;4:37–68. doi: 10.1146/annurev.iy.04.040186.000345. [DOI] [PubMed] [Google Scholar]
- Barnaba V., Zaccari C., Levrero M., Balsano F. Suppressor T cells role in the unresponsiveness to Candida albicans in chronic mucocutaneous candidiasis. Boll Ist Sieroter Milan. 1985;64(2):126–130. [PubMed] [Google Scholar]
- Borkowsky W., Valentine F. T. The proliferative response of human lymphocytes to antigen is suppressed preferentially by lymphocytes precultured with the same antigen. J Immunol. 1979 May;122(5):1867–1873. [PubMed] [Google Scholar]
- Canales L., Middlemas R. O., 3rd, Louro J. M., South M. A. Immunological observations in chronic mucocutaneous candidiasis. Lancet. 1969 Sep 13;2(7620):567–571. doi: 10.1016/s0140-6736(69)90264-5. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Regulation of cellular and humoral immune responses by T-cell subclasses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):23–32. doi: 10.1101/sqb.1977.041.01.006. [DOI] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff C. F., Packer B., Rivas V., Rogers C. M., Cassone A., Donnelly R., Rogers T. J. Induction of immunosuppressive B-lymphocytes with components of Candida albicans. Adv Exp Med Biol. 1988;239:367–378. doi: 10.1007/978-1-4757-5421-6_35. [DOI] [PubMed] [Google Scholar]
- Cuff C. F., Rogers C. M., Lamb B. J., Rogers T. J. Induction of suppressor cells in vitro by Candida albicans. Cell Immunol. 1986 Jun;100(1):47–56. doi: 10.1016/0008-8749(86)90005-5. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Childs A. L., Doyle L. V. Immunoregulatory T lymphocytes in man. Soluble antigen-specific suppressor-inducer T lymphocytes are derived from the CD4+CD45R-p80+ subpopulation. J Immunol. 1987 Sep 1;139(5):1501–1508. [PubMed] [Google Scholar]
- Domer J. E., Garner R. E., Befidi-Mengue R. N. Mannan as an antigen in cell-mediated immunity (CMI) assays and as a modulator of mannan-specific CMI. Infect Immun. 1989 Mar;57(3):693–700. doi: 10.1128/iai.57.3.693-700.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Hector R. F. Enhanced immune responses in mice treated with penicillin-tetracycline or trimethoprim-sulfamethoxazole when colonized intragastrically with Candida albicans. Antimicrob Agents Chemother. 1987 May;31(5):691–697. doi: 10.1128/aac.31.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Intragastric colonization of infant mice with Candida albicans induces systemic immunity demonstrable upon challenge as adults. J Infect Dis. 1988 May;157(5):950–958. doi: 10.1093/infdis/157.5.950. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Specific in vitro antimannan-rich antigen of Candida albicans antibody production by sensitized human blood lymphocytes. J Clin Invest. 1983 Jun;71(6):1602–1613. doi: 10.1172/JCI110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Le Deist F., Drouhet E., Griscelli C. Mannan-specific and mannan-induced T-cell suppressive activity in patients with chronic mucocutaneous candidiasis. J Clin Immunol. 1987 Sep;7(5):400–409. doi: 10.1007/BF00917018. [DOI] [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Pichat L., Audinot M., Griscelli C. Defective handling of mannan by monocytes in patients with chronic mucocutaneous candidiasis resulting in a specific cellular unresponsiveness. Clin Exp Immunol. 1982 Mar;47(3):653–660. [PMC free article] [PubMed] [Google Scholar]
- Garcia-de-Lomas J., Morales C., Grau M. A., Mir A. Detection of Candida sp. mannan antigen by indirect ELISA-inhibition. In vitro crossed reactivity among mannans obtained from C. albicans A, C. albicans B, and C. tropicalis. Mycopathologia. 1988 Jun;102(3):175–178. doi: 10.1007/BF00437401. [DOI] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R., Flood P. M., Gershon R. K. Immunoregulatory T-cell pathways. Annu Rev Immunol. 1983;1:439–463. doi: 10.1146/annurev.iy.01.040183.002255. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O., LOEWE J. Antigenic studies of Candida. II. Antigenic relation of Candida albicans group A and group B to Candida stellatoidea and Candida tropicalis. J Bacteriol. 1961 Oct;82:574–577. doi: 10.1128/jb.82.4.574-577.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Finkel B. E., Murphy J. W. Characterization of efferent T suppressor cells induced by Paracoccidioides brasiliensis-specific afferent T suppressor cells. Infect Immun. 1988 Apr;56(4):744–750. doi: 10.1128/iai.56.4.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Finkel B. E., Murphy J. W. Induction of antigen-specific T suppressor cells by soluble Paracoccidioides brasiliensis antigen. Infect Immun. 1988 Apr;56(4):734–743. doi: 10.1128/iai.56.4.734-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce F. M., Mills D. M., Iverson K., Cousins R., Everett E. D. Inhibition of leukocyte candidacidal activity by serum from patients with disseminated candidiasis. J Lab Clin Med. 1975 Oct;86(4):657–666. [PubMed] [Google Scholar]
- Lehner T., Wilton J. M., Ivanyi L. Immunodeficiencies in chronic muco-cutaneous candidosis. Immunology. 1972 May;22(5):775–787. [PMC free article] [PubMed] [Google Scholar]
- Manohar V., Brown E., Leiserson W. M., Chused T. M. Expression of Lyt-1 by a subset of B lymphocytes. J Immunol. 1982 Aug;129(2):532–538. [PubMed] [Google Scholar]
- Moser S. A., Domer J. E., Mather F. J. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1980 Jan;27(1):140–149. doi: 10.1128/iai.27.1.140-149.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Paterson P. Y., Semo R., Blumenschein G., Swelstad J. Mucocutaneous candidiasis, anergy and a plasma inhibitor of cellular immunity: reversal after amphotericin B therapy. Clin Exp Immunol. 1971 Nov;9(5):595–602. [PMC free article] [PubMed] [Google Scholar]
- Pavliak V., Sandula J. Cross-reactivity of pathogenic Candida mannans studied by enzyme-linked immunosorbent assay (ELISA) and precipitin methods. Mycoses. 1988 Jan;31(1):34–39. [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGI S. J., DI LUZIO N. R. Identification of a reticuloendothelial stimulating agent in zymosan. Am J Physiol. 1961 Feb;200:297–300. doi: 10.1152/ajplegacy.1961.200.2.297. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., McKenzie I. F., Bretscher P. A., Parish C. R. Discrimination of suppressor T cells of humoral and cell-mediated immunity by anti-Ly and anti-Ia sera. Cell Immunol. 1977 Jun 15;31(2):364–369. doi: 10.1016/0008-8749(77)90038-7. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas V., Rogers T. J. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983 Jan;130(1):376–379. [PubMed] [Google Scholar]
- Taub D. D., Lin Y. S., Rogers T. J. Characterization and genetic restriction of suppressor-effector cells induced by staphylococcal enterotoxin B. J Immunol. 1990 Jan 15;144(2):456–462. [PubMed] [Google Scholar]
- Thompson C. H., Potter T. A., McKenzie I. F., Parish C. R. The surface phenotype of a suppressor cell of delayed-type hypersensitivity in the mouse. Immunology. 1980 May;40(1):87–96. [PMC free article] [PubMed] [Google Scholar]
- WOOLES W. R., DILUZIO N. R. RETICULOENDOTHELIAL FUNCTION AND THE IMMUNE RESPONSE. Science. 1963 Nov 22;142(3595):1078–1080. doi: 10.1126/science.142.3595.1078. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Yu I. R., Ledger W. J. Inhibition of Candida albicans--induced lymphocyte proliferation by lymphocytes and sera from women with recurrent vaginitis. Am J Obstet Gynecol. 1983 Dec 1;147(7):809–811. doi: 10.1016/0002-9378(83)90044-3. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Bowie J. U., Nelson R. D. Influence of yeast mannan on release of myeloperoxidase by human neutrophils: determination of structural features of mannan required for formation of myeloperoxidase-mannan-neutrophil complexes. Infect Immun. 1984 Feb;43(2):467–471. doi: 10.1128/iai.43.2.467-471.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


