Abstract
Purpose
To estimate the mean rates of ocular function loss in patients with autosomal recessive retinitis pigmentosa due to USH2A mutations.
Methods
In 125 patients with USH2A mutations, we used longitudinal regression to estimate mean rates of change of Snellen visual acuity, Goldmann visual field area (V4e white test light), and 30 Hz (cone) full-field electroretinogram amplitude. We compared these rates to those of previously studied cohorts with dominant retinitis pigmentosa due to RHO mutations and with X-linked retinitis pigmentosa due to RPGR mutations. We also compared rates of change in patients with the Cys759Phe mutation, the USH2A mutation associated with nonsyndromic disease, with rates of change in patients with the Glu767fs mutation, the most common USH2A mutation associated with Usher syndrome, type II (i.e., retinitis pigmentosa and hearing loss).
Results
Mean annual exponential rates of decline for the USH2A patients were 2.6% for visual acuity, 7.0% for visual field area, and 13.2% for electroretinogram amplitude. The rate of acuity loss fell between the corresponding rates for the RHO and RPGR patients, while the rates for field and ERG amplitude loss were faster than those for the RHO and RPGR patients. No significant differences were found for patients with the Cys759Phe mutation versus patients with the Glu767fs mutation.
Conclusions
On average, USH2A patients lose visual acuity faster than RHO patients and slower than RPGR patients. USH2A patients lose visual field and cone electroretinogram amplitude faster than RHO patients and RPGR patients. Patients with a nonsyndromic USH2A mutation have the same retinal disease course as patients with syndromic USH2A disease.
Introduction
Mutations in the genes encoding rhodopsin (RHO), retinitis pigmentosa GTPase regulator (RPGR), and usherin (USH2A) are the most common causes of retinitis pigmentosa, each gene accounting for about 10% of cases in North America.1-3 We previously reported differences in the mean rates of loss of visual acuity, visual field, and ERG amplitude between patients with RHO mutations and patients with RPGR mutations.4 The present study was done to determine the rates of progression for patients with USH2A mutations and compare them to those previously reported for the other two common forms of retinitis pigmentosa.
In 1998 mutations in the USH2A gene were first reported as a cause for Usher syndrome, type II, an autosomal recessive form of retinitis pigmentosa with mild-to-moderate congenital hearing loss and normal vestibular function,5 based on analysis of the 21 exons found to encode an USH2A transcript.6 A longer transcript consisting of 51 additional 3’ exons was more recently identified, and mutations causing Usher syndrome, type II were also found in these exons.7 Most USH2A mutations individually account for a only few percent of cases of Usher syndrome, type II. An exception is the mutation c.2299delG (Glu767fs) in exon 13 which is geographically widespread and found to have been derived from a common ancestor.8 In addition, another common mutation in this gene—Cys759Phe, also in exon 13 —has been reported to cause 4% to 5% of autosomal recessive retinitis pigmentosa without hearing loss.9,10 Because in many cases this mutation in one allele does not cause hearing loss even when combined with a pathogenic USH2A mutation in the second allele,9,10 we hypothesized that a patient with at least one allele carrying the Cys759Phe mutation might have a less severe ocular disease course than patients with other USH2A mutations. To test this hypothesis, we also compared mean rates of decline of patients with the Cys759Phe mutation to those of patients with the Glu767fs mutation. We restricted this comparison to patients who carried the one mutation without carrying the other mutation.
Methods
Patients
This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of the Massachusetts Eye and Ear Infirmary and Harvard Medical School. Our USH2A longitudinal dataset comprised 125 patients with pathogenic mutations (see Table 2). This cohort (mean age at baseline: 31.6 years, age range at baseline: 6 to 59 years) had 3 to 27 years of follow-up from 1975 to 2005 utilizing the same test conditions with a mean follow-up of 10.4 years and an average of 7.4 ocular examinations/patient. All patients had parents with no history of retinitis pigmentosa, and, therefore, we presumed that the condition in these patients was autosomal recessive. The RHO and RPGR longitudinal datasets, derived from a previous study,2 were also limited to patients with at least 3 years of follow-up. They yielded a sample of 134 RHO patients (mean age at baseline: 36.0 years, age range at baseline: 8 to 66 years) who had been followed for 3 to 24 years with an average follow-up of 8.9 years based on an average of 6.2 examinations/patient and 113 RPGR patients (mean age at baseline: 26.1 years, age range at baseline: 5 to 61 years) who had been followed for 3 to 28 years with an average follow-up of 9.8 years based on an average of 7.2 examinations/patient. The mean age at baseline of the patients varied significantly by group (p < 0.001).
Table 2.
Baseline Ocular Function of Patients with USH2A Mutations
| ID | Mutation (Exon) | Mutation (Exon) | Age | VA OD* | VA OS* | VF OD† | VF OS† | ERG OD‡ | ERG OS‡ |
|---|---|---|---|---|---|---|---|---|---|
| 19238 | Lys182fs (3) | Glu767fs (13) | 24 | 20/100 | 20/70 | 6438 | 5205 | 0.61 | 0.48 |
| 22085 | His308fs (6) | Trp3955X (61) | 59 | 20/200 | 20/200 | 152 | 213 | 0.30 | 0.15 |
| 19083 | His308fs (6) | 15 | 20/30 | 20/30 | 10451 | 8768 | 1.89 | 1.82 | |
| 06863 | Ser343fs (6) | 34 | 20/50 | 20/60 | 384 | 279 | 0.30 | 0.30 | |
| 02933 | Ile371fs (6) | 31 | 20/30 | 20/40 | 1073 | 585 | NA | NA | |
| 19824 | Asn405fs (7) | 26 | 20/30 | 20/30 | 3795 | 3457 | 0.73 | 0.61 | |
| 06236 | Cys419Phe (7) | 28 | 20/40 | 20/40 | 14349 | 15336 | 3.04 | 3.72 | |
| 19477 | Cys419Phe (7) | IVS10-2A>G (I10) | 44 | 20/40 | 20/40 | 410 | 545 | 0.08 | 0.31 |
| 00304 | Cys419Phe (7) | 35 | 20/25 | 20/25 | 10716 | 10322 | 6.00 | 6.00 | |
| 07018 | Cys419Phe (7) | 30 | 20/30 | 20/30 | 16134 | 15672 | 2.86 | 2.62 | |
| 13287 | Arg626X (11) | Arg626X (11) | 32 | 20/25 | 20/25 | 3700 | 3610 | 0.23 | 0.09 |
| 15597 | Arg626X (11) | Cys1447fs (20) | 20 | 20/30 | 20/30 | NA | NA | 2.31 | 1.75 |
| 13574 | Cys691X (12) | Glu767fs (13) | 25 | 20/20 | 20/20 | 8157 | 7770 | 0.90 | 0.90 |
| 14419 | Thr701fs (12) | Gly3142X (48) | 17 | 20/20 | 20/30 | 6926 | 4379 | 0.45 | 0.37 |
| 01376 | IVS12-1G>C (I12) | 53 | 20/30 | 20/40 | NA | NA | NA | NA | |
| 05831 | Cys759Phe (13) | 43 | 20/40 | 20/50 | 1576 | 1646 | 0.26 | 0.24 | |
| 13445 | Cys759Phe (13) | Trp3955X (61) | 28 | 20/30 | 20/30 | NA | NA | 29.40 | 29.40 |
| 15657 | Cys759Phe (13) | Cys759Phe (13) | 36 | 20/25 | 20/50 | 10920 | 9497 | 9.24 | 4.20 |
| 11572 | Cys759Phe (13) | Cys759Phe (13) | 15 | 20/30 | 20/30 | 7711 | 9867 | 0.41 | 0.55 |
| 05014 | Cys759Phe (13) | Cys759Phe (13) | 35 | 20/40 | 20/30 | 9132 | 8980 | 22.00 | 22.00 |
| 06705 | Cys759Phe (13) | Glu767fs (13) | 28 | 20/25 | 20/25 | 21176 | 23087 | 13.74 | 12.71 |
| 05918 | Cys759Phe (13) | Glu767fs (13) | 40 | 20/30 | 20/30 | 8367 | 8174 | 7.48 | 9.13 |
| 06475 | Cys759Phe (13) | Glu767fs (13) | 32 | 20/40 | 20/40 | 1556 | 1117 | 2.07 | 1.83 |
| 06792 | Cys759Phe (13) | Cys1447fs (20) | 33 | 20/20 | 20/20 | 4288 | 3258 | 3.19 | 2.36 |
| 07889 | Cys759Phe (13) | Cys1447fs (20) | 27 | 20/20 | 20/20 | 8243 | 7725 | 29.40 | 52.94 |
| 11439 | Cys759Phe (13) | Cys1447fs (20) | 28 | 20/20 | 20/20 | 10077 | 11732 | 10.64 | 14.00 |
| 14338 | Cys759Phe (13) | Pro1978fs (30) | 36 | 20/20 | 20/25 | 7600 | 7944 | 0.20 | 0.11 |
| 14436 | Cys759Phe (13) | IVS29+2T>C (I29) | 43 | 20/20 | 20/40 | 12170 | 12186 | 23.50 | 20.60 |
| 14483 | Cys759Phe (13) | 38 | 20/20 | 20/20 | 6934 | 6403 | 29.40 | 23.50 | |
| 06966 | Cys759Phe (13) | 44 | 20/30 | 20/30 | 4245 | 4077 | 11.69 | 8.06 | |
| 05666 | Cys759Phe (13) | 44 | 20/20 | 20/20 | 9554 | 9787 | 1.20 | 1.90 | |
| 03458 | Cys759Phe (13) | Glu4671fs (64) | 34 | 20/25 | 20/25 | 4703 | 4488 | NA | NA |
| 15221 | Cys759Phe (13) | 34 | 20/20 | 20/20 | 8843 | 8927 | 7.79 | 11.40 | |
| 19431 | Cys759Phe (13) | 21 | 20/30 | 20/30 | 15970 | 16092 | 19.6 | 18.2 | |
| 06191 | Cys759Phe (13) | 33 | 20/30 | 20/30 | 2751 | 3039 | 0.09 | 0.21 | |
| 05917 | Cys759Phe (13) | 38 | 20/20 | 20/25 | 17802 | 20246 | 5.08 | 4.99 | |
| 05907 | Cys759Phe (13) | 35 | 20/25 | 20/25 | 5312 | 4899 | 1.89 | 1.32 | |
| 07008 | Cys759Phe (13) | 43 | 20/60 | 20/100 | 196 | 231 | 0.16 | 0.13 | |
| 06898 | Cys759Phe (13) | 40 | 20/30 | 20/30 | 9763 | 9364 | 1.13 | 2.62 | |
| 05967 | Cys759Phe (13) | 33 | 20/20 | 20/20 | 402 | 583 | 1.21 | 0.77 | |
| 03753 | Cys759Phe (13) | 33 | 20/30 | 20/50 | 12960 | 10952 | 59.0 | 59.0 | |
| 02874 | Cys759Phe (13) | 33 | 20/25 | 20/25 | 10191 | 10172 | 6.00 | 6.00 | |
| 07065 | Cys759Phe (13) | 45 | 20/30 | 20/30 | 16342 | 17005 | 6.25 | 6.12 | |
| 00220 | Cys759Phe (13) | 40 | 20/25 | 20/20 | 10102 | 9972 | NA | NA | |
| 05377 | Cys759Phe (13) | 41 | 20/25 | 20/30 | 8560 | 9000 | 14.0 | 8.40 | |
| 06144 | Cys759Phe (13) | 38 | 20/30 | 20/25 | 1572 | 490 | 0.33 | 0.18 | |
| 06210 | Cys759Phe (13) | 37 | 20/50 | 20/60 | 2555 | 1449 | 1.80 | 2.08 | |
| 06732 | Cys759Phe (13) | 32 | 20/30 | 20/30 | 175 | 227 | 3.41 | 2.54 | |
| 07879 | Cys759Phe (13) | 45 | 20/40 | 20/30 | 3148 | 3377 | 23.5 | 11.00 | |
| 13613 | Cys759Phe (13) | 26 | 20/25 | 20/25 | 98 | 733 | 1.30 | 2.50 | |
| 11259 | Cys759Phe (13) | 28 | 20/20 | 20/20 | 6154 | 8217 | 1.40 | 5.04 | |
| 11505 | Cys759Phe (13) | 25 | 20/25 | 20/25 | 8759 | 7407 | 1.50 | 0.52 | |
| 05011 | Cys759Phe (13) | 33 | 20/20 | 20/20 | 11992 | 10804 | 11.00 | 21.0 | |
| 13115 | Cys759Phe (13) | 44 | 20/20 | 20/20 | 8155 | 9740 | 1.20 | 1.90 | |
| 14027 | Cys759Phe (13) | 32 | 20/25 | 20/20 | 13887 | 12868 | 23.50 | 23.50 | |
| 02726 | Cys759Phe (13) | 55 | 20/30 | 20/30 | 7391 | 6701 | 11.00 | 11.00 | |
| 02943 | Cys759Phe (13) | 23 | 20/30 | 20/25 | 9294 | 8715 | NA | NA | |
| 02954 | Cys759Phe (13) | 45 | 20/20 | 20/20 | 1001 | 1460 | NA | 12.0 | |
| 00994 | Cys759Phe (13) | 36 | 20/40 | 20/50 | 366 | 264 | NA | NA | |
| 15169 | Glu767fs (13) | 17 | 20/25 | 20/25 | 7686 | 8028 | 0.35 | 0.21 | |
| 11451 | Glu767fs (13) | Gln4960fs (68) | 27 | 20/20 | 20/20 | 9997 | 10274 | 1.19 | 1.40 |
| 22045 | Glu767fs (13) | Glu767fs (13) | 27 | 20/50 | 20/50 | 6141 | 4992 | 1.87 | NA |
| 02265 | Glu767fs (13) | Cys1447fs (20) | 17 | 20/25 | 20/50 | 5415 | 5654 | NA | NA |
| 02267 | Glu767fs (13) | Cys1447fs (20) | 16 | 20/25 | 20/30 | 7430 | 7106 | NA | NA |
| 02266 | Glu767fs (13) | Cys1447fs (20) | 15 | 20/20 | 20/20 | 10467 | 9806 | 62.0 | NA |
| 19249 | Glu767fs (13) | Arg1549X (22) | 22 | 20/20 | 20/20 | 3802 | 2508 | 0.80 | 0.53 |
| 15499 | Glu767fs (13) | Arg2853X (42) | 24 | 20/30 | 20/30 | 6201 | 7648 | 0.80 | 0.88 |
| 05958 | Glu767fs (13) | 37 | 20/30 | 20/30 | 12011 | 12341 | 8.00 | 8.00 | |
| 11300 | Glu767fs (13) | IVS29+1G>C (I29) | 45 | 20/40 | 20/40 | 290 | 523 | 0.20 | 0.20 |
| 19281 | Glu767fs (13) | IVS61-2A>G (I61) | 44 | 20/25 | 20/20 | 3329 | 1618 | 2.35 | 1.91 |
| 06991 | Glu767fs (13) | 40 | 20/25 | 20/30 | 9672 | 12803 | 0.96 | 0.93 | |
| 06724 | Glu767fs (13) | 31 | 20/40 | 20/40 | 12591 | 11592 | 1.10 | 0.92 | |
| 05148 | Glu767fs (13) | 40 | 20/30 | 20/200 | 8558 | 7642 | 2.72 | 1.79 | |
| 15297 | Glu767fs (13) | 45 | 20/60 | 20/25 | 11342 | 10503 | 5.87 | 4.56 | |
| 15375 | Glu767fs (13) | 47 | 20/25 | 20/25 | 11456 | 11815 | 1.98 | 1.44 | |
| 06704 | Glu767fs (13) | 24 | 20/30 | 20/40 | 10999 | 7670 | 35.9 | 34.2 | |
| 06750 | Glu767fs (13) | 32 | 20/30 | 20/40 | 3447 | 2591 | 4.32 | 4.02 | |
| 06178 | Glu767fs (13) | 36 | 20/40 | 20/25 | 650 | 881 | 0.56 | 0.48 | |
| 06226 | Glu767fs (13) | 26 | 20/30 | 20/30 | 8477 | 9762 | 1.25 | 1.42 | |
| 06256 | Glu767fs (13) | Arg1549X (22) | 27 | 20/25 | 20/30 | 3900 | 4100 | 0.31 | 0.33 |
| 06650 | Glu767fs (13) | 33 | 20/20 | 20/25 | 13164 | 11544 | 1.62 | 1.81 | |
| 06666 | Glu767fs (13) | 23 | 20/25 | 20/25 | 944 | 888 | 0.51 | 0.85 | |
| 05751 | Glu767fs (13) | 23 | 20/30 | 20/20 | 5306 | 5518 | 0.32 | 0.47 | |
| 06017 | Glu767fs (13) | 31 | 20/25 | 20/25 | 4705 | 3610 | 5.37 | 4.65 | |
| 05916 | Glu767fs (13) | 39 | 20/25 | 20/30 | 4140 | 5787 | 1.96 | 1.96 | |
| 03960 | Glu767fs (13) | 17 | 20/40 | 20/30 | 11182 | 10842 | 1.04 | 0.77 | |
| 06798 | Glu767fs (13) | 32 | 20/25 | 20/25 | 14005 | 15158 | 2.05 | 1.38 | |
| 05556 | Glu767fs (13) | 19 | 20/25 | 20/25 | 11028 | 15047 | 0.26 | 0.35 | |
| 05213 | Glu767fs (13) | 15 | 20/20 | 20/20 | 7774 | 8778 | 1.05 | 1.08 | |
| 02130 | Glu767fs (13) | 21 | 20/20 | 20/25 | 3122 | 3561 | 0.94 | 0.94 | |
| 07031 | Glu767fs (13) | 26 | 20/30 | 20/40 | 14551 | 13448 | 1.15 | 0.77 | |
| 02510 | Glu767fs (13) | 26 | 20/30 | 20/25 | 565 | 2407 | 6.00 | 6.00 | |
| 03193 | Glu767fs (13) | 28 | 20/20 | 20/20 | 638 | 690 | NA | NA | |
| 05800 | Glu767fs (13) | 42 | 20/50 | 20/50 | 1798 | 2151 | 0.22 | 0.34 | |
| 05785 | Glu767fs (13) | 27 | 20/30 | 20/25 | 14130 | 17982 | 3.52 | 3.07 | |
| 07401 | Glu767fs (13) | 39 | 20/40 | 20/30 | 283 | 332 | 0.16 | 0.16 | |
| 05252 | Glu767fs (13) | Arg4935X (68) | 42 | NA | NA | 9897 | 9504 | NA | NA |
| 14015 | Glu767fs (13) | 25 | 20/20 | 20/20 | 9755 | 10317 | 17.60 | 20.60 | |
| 01445 | Glu767fs (13) | 54 | 20/30 | 20/30 | 10561 | 10759 | 0.25 | 0.67 | |
| 15938 | Glu767fs (13) | 29 | 20/30 | 20/40 | 7220 | 7556 | 0.74 | 0.60 | |
| 15579 | Glu767fs (13) | 31 | 20/25 | 20/25 | 8868 | 6637 | 1.12 | 0.87 | |
| 19822 | Glu767fs (13) | 52 | 20/40 | 20/60 | 168 | 138 | 0.14 | 0.11 | |
| 05766 | Leu921fs (13) | Cys1447fs (20) | 30 | 20/25 | 20/20 | 973 | 1400 | 0.49 | 0.68 |
| 15285 | Gln1063fs (16) | Trp2945X (44) | 22 | 20/25 | NA | 6457 | 4376 | NA | NA |
| 05960 | Gln1408X (19) | 33 | 20/25 | 20/25 | 3974 | 5820 | NA | NA | |
| 07916 | Ile1439fs (20) | 30 | 20/20 | 20/20 | NA | NA | 0.50 | 0.70 | |
| 04129 | Cys1447fs (20) | 6 | 20/40 | 20/30 | 10261 | 11787 | NA | NA | |
| 04032 | Cys1447fs (20) | 7 | 20/40 | 20/30 | 4839 | 4754 | NA | NA | |
| 03482 | Cys1447fs (20) | 19 | 20/30 | 20/30 | 10576 | 11651 | NA | NA | |
| 06241 | Cys1447fs (20) | 36 | 20/30 | 20/30 | 13131 | 7372 | 11.96 | 12.59 | |
| 07607 | Cys1447fs (20) | Arg4935X (68) | 15 | 20/20 | 20/20 | 10117 | 10547 | NA | 1.40 |
| 11719 | Cys1447fs (20) | Cys1447fs (20) | 27 | 20/25 | 20/25 | 9121 | 9317 | 1.10 | 1.10 |
| 06019 | Cys1447fs (20) | 40 | 20/50 | 20/50 | 489 | 421 | 0.10 | 0.12 | |
| 06088 | Arg1504fs (21) | 20 | 20/25 | 20/25 | 1572 | 1410 | 0.20 | 0.30 | |
| 03811 | Arg1549X (22) | 31 | 20/30 | 20/40 | 2925 | 3961 | NA | NA | |
| 05857 | Met1731fs (26) | 39 | 20/70 | 20/100 | 14753 | 14331 | 3.14 | 2.50 | |
| 07278 | Gln2057X (32) | 22 | 20/20 | 20/30 | 8943 | 10503 | 0.60 | 0.20 | |
| 05558 | Ser2498fs (40) | 34 | 20/25 | 20/30 | 11524 | 10354 | 3.02 | 2.42 | |
| 14888 | Arg2723X (41) | Arg2723X (41) | 43 | 20/400 | 20/80 | 186 | 172 | 0.10 | NA |
| 00943 | Arg2723X (41) | 36 | 20/50 | HM | 234 | 198 | 0.17 | 0.10 | |
| 03541 | Gly3142X (48) | 29 | 20/30 | 20/25 | 434 | 347 | NA | NA | |
| 04132 | Lys3397fs (52) | IVS28+1G>A (I28) | 30 | HM | 20/50 | NA | 8261 | NA | NA |
| 14402 | Gln3845X (59) | Gln3845X (59) | 18 | 20/30 | 20/25 | 4605 | 3266 | 0.23 | 0.16 |
| 05765 | Trp3955X (61) | 24 | 20/30 | 20/50 | 110 | 129 | 0.29 | 0.33 | |
| 14518 | Gln4711X (64) | 22 | 20/20 | 20/25 | 12843 | 13452 | 4.90 | 3.60 | |
| Arithmetic mean | 31.6 | 20/28 | 20/29 | 7003 | 6987 | 5.91 | 5.50 | ||
| Number of patients with values | 125 | 124 | 123 | 120 | 121 | 105 | 104 |
Parentheses enclosing an “I” designate “intron”.
Measured with a projected Snellen chart.
Visual field area in deg2 to the V4e white stimulus of the Goldmann perimeter (normal ≥ 11310 deg2).
ERG amplitude in μV to 30 Hz white full-field stimulation (normal ≥ 50 μV).
“NA” = not available.
Clinical evaluation
All of the patients underwent identical ocular examinations and quantification of data as described in detail previously.4 We recorded best-corrected Snellen visual acuities and coded them as decimals. Kinetic visual fields were measured to the V4e white test light against the standard background of 31.5 apostilbs. Fields were plotted with a digitizing tablet or scanned by custom software and converted to areas in deg2. We placed a contact lens electrode on the topically anesthetized cornea and elicited full-field cone ERGs with 10 μs, 30 Hz flashes of white light (0.2 cd-s/m2) after pupillary dilation and 45 minutes of dark adaptation; digital filtering and signal averaging were used to quantify responses < 10 μV in amplitude. As part of another research project,11 we had recorded optical coherence tomograms (OCTs) from 4 of the patients with the Glu767fs mutation (ages, 43 to 54 years) who had visual acuities spanning 20/20 to 20/70. We evaluated these tomograms to try to reveal the morphological basis for visual acuity loss in this disease.
Statistical analyses
We censored visual acuities, visual field areas, and ERG amplitudes from selected visits to minimize ceiling and floor effects, converted these measures to natural logarithms, and performed analyses as described previously.4 In addition, we excluded USH2A patients from visual field analyses if they showed marked inconsistency over the course of follow-up, possibly due to an impaired ability to hear the examiner’s prompts. Specifically, we eliminated patients if their root mean square error from the regression of loge visual field area on time exceeded 4 standard deviations from the grand mean. Altogether, we excluded 23 patients from the analysis of visual acuity change, 16 patients from the analysis of visual field change, and 56 patients from the analysis of ERG change.
We used repeated-measures longitudinal regression with PROC MIXED of SAS, version 9 (SAS Institute, Cary, NC) to estimate the mean rate of change for each outcome measure based on the average loge value for one or both eyes at each visit and compared mean slopes for patients by genotype. We used PROC LIFEREG of SAS to fit a Weibull function to survival data to compare the age distribution for legal blindness (i.e., a visual acuity of 20/200 or worse or a visual field diameter of 20° or less in each eye) in patients by genotype. This procedure accounts for left censoring (i.e., patients who were legally blind at their baseline visit), right censoring (i.e., patients who had not become legally blind by their last follow-up visit), and interval censoring (i.e., patients who became legally-blind between two follow-up visits).
Results
USH2A Mutations
Table 1 lists the nucleotide change and protein change for each of the 38 mutations identified in the patients included in this study. Many of these mutations were reported previously as the result of a screen of the short isoform of USH2A.3 Subsequently, we screened additional patients including the exons for the long isoform and found 18 novel mutations (indicated in bold).
Table 1.
USH2A Mutations in DNA and Protein Format
| Exon/Intron | Nucleotide change | Protein change |
|---|---|---|
| 3 | c.545_546delAA | Lys182fs |
| 6 | c.920_923dupGCCA | His308fs |
| 6 | c.1026_29delCTCT | Ser343fs* |
| 6 | c.1110_1111delTA | Ile371fs |
| 7 | c.1214delA | Asn405fs |
| 7 | c.1256G>T | Cys419Phe |
| Intron 10 | c.1841-2A>G | Alters splice site IVS10-2A>G |
| 11 | c.1876C>T | Arg626X |
| 12 | c.2073C>A | Cys691X |
| 12 | c.2100delG | Thr701fs* |
| Intron 12 | c.2168-1G>C | Alters splice site IVS12-1G>C |
| 13 | c.2276G>T | Cys759Phe |
| 13 | c.2299delG | Glu767fs |
| 13 | c.2761del C | Leu921fs |
| 16 | c.3187_3188delCA | Gln1063fs |
| 20 | c.4314delG | Ile1439fs |
| 20 | c.4338_39delCT | Cys1447fs |
| 21 | c.4510_4511insA | Arg1504fs |
| 22 | c.4645C>T | Arg1549X |
| 26 | c.5191_5192delAT | Met1731fs |
| Intron 28 | c.5775+1G>A | Alters splice site IVS28+1G>A |
| Intron 29 | c.5857+1G>C | Alters splice site IVS29+1G>C |
| Intron 29 | c.5857+2T>C | Alters splice site IVS29+2T>C |
| 30 | c.5933_5940delCTGTTGTC | Pro1978fs |
| 32 | c.6169C>T | Gln2057X |
| 40 | c.7493delG | Ser2498fs |
| 41 | c.8167C>T | Arg2723X |
| 42 | c.8557A>T | Arg2853X |
| 44 | c.8834G>A | Trp2945X |
| 48 | c.9424G>T | Gly3142X |
| 52 | c.10190_10191delAA | Lys3397fs |
| 59 | c.11533C>T | Gln3845X |
| 61 | c.11864G>A | Trp3955X |
| Intron 61 | c.12067-2A>G | Alters splice site IVS61-2A>G |
| 64 | c.14010_14062del | Glu4671fs |
| 64 | c.14131C>T | Gln4711X |
| 68 | c.14803C>T | Arg4935X |
| 68 | c.14879_c.14880delAAins45 | Gln4960fs |
Mutations in bold are novel.
Mutation nomenclature in original publication3 was incorrect.
Table 2 lists the identified mutations by patient. Not all patients were screened for mutations in the entire gene, and some patients carried a rare USH2A missense change that was not demonstrably pathogenic; these factors likely account for the high prevalence of patients with only one detected pathogenic mutation in our series. The table shows that 41 patients (33%) had the Cys759Phe mutation, associated with nonsyndromic retinitis pigmentosa, and not the Glu767fs mutation, associated with hearing loss. Conversely, 43 patients (34%) had the Glu767fs mutation and not the Cys759Phe mutation.
Baseline ocular function
Table 2 also reveals that at a mean age of 32 years our USH2A patients had mean visual acuities that were reduced 1 to 2 lines, mean visual fields that were reduced about 40% below normal, and cone ERG amplitudes that were reduced nearly 90% below normal.
Mean rates of change
Table 3 lists the mean annual loge rates of change for patients with USH2A mutations with standard errors and significance levels. The mean loge values correspond to mean annual exponential rates of decline of 2.6% for Snellen visual acuity, 7.0% for visual field area to the V4e test light, and 13.2% for cone ERG amplitude to 30 Hz flashes. In comparison, the RHO patients and RPGR patients, respectively, had mean annual exponential rates of decline of 1.6% and 4.0% for visual acuity, 2.9% and 4.7% for visual field area, and 7.7% and 7.1% for cone ERG amplitude. The mean rate of visual acuity loss for the USH2A patients was significantly faster than that for the RHO patients (p = 0.005) but significantly slower than that for the RPGR patients (p < 0.001). On the other hand, the mean rate of visual field loss and the mean rate of ERG amplitude loss for the USH2A patients were significantly faster than the corresponding rates for the RHO patients and for the RPGR patients (p < 0.001 in all cases).
Table 3.
Annual Rates of Change in Patients with USH2A Mutations
| Ocular Function | N* | Mean ± SEM (geometric mean)† | P-value† |
|---|---|---|---|
| Loge Visual Acuity | 102 | -0.026± 0.002 (-2.6%) | < 0.001 |
| Loge Visual Field Area | 109 | -0.074± 0.003 (-7.0%) | < 0.001 |
| Loge ERG Amplitude | 69 | -0.141± 0.005 (-13.2%) | < 0.001 |
After censoring data to minimize ceiling and floor effects and preserve a follow-up ≥ 3 years (see Methods for details).
Calculated by longitudinal regression using PROC MIXED of SAS.
We then compared mean rates of change for USH2A patients with the Cys759Phe mutation (and not the Glu767fs mutation) to the mean rates for USH2A patients with the Glu767fs mutation (and not the Cys759Phe mutation). Mean rates of annual decline were, respectively, 2.9% versus 2.1% for visual acuity, 6.9% versus 7.7% for visual field area, and 13.1% versus 12.9% for ERG amplitude. None of these differences was statistically significant.
Distributions of rate of change
Figures 1 to 3 show the distributions of annual rate of change by ocular function and genotype. The x-axes are all spaced in intervals of 0.1 loge-unit (i.e., ~10%) and, for a given measure of ocular function, span the same range. The modal values provide a gauge of the variation in rate of change and represent 60.6% to 61.8% of cases for visual acuity, 47.6% to 54.7% of cases for visual field area, and 33.3% to 40.4% of cases for cone ERG amplitude. Even though these modal values, on average, represent only about half of each group of change scores, it is still possible to discern differences in these distributions that parallel the mean differences cited above. With the location of the mode on the x-axis for the USH2A patients as reference, the cumulative percentage of cases to the left of this value (i.e., associated with faster progression) for visual acuity is 9.9% for USH2A patients, 6.7% for RHO patients, and 19.5% for RPGR patients. The cumulative percentage of cases to the left of the USH2A mode for visual field area is 31.2% for USH2A patients, 11% for RHO patients, and 19.5% for RPGR patients. The cumulative percentage of cases to the left of the USH2A mode for cone ERG amplitude is 37.6% for USH2A patients, 12.3% for RHO patients, and 15.1% for RPGR patients. Thus, the visual acuity distribution for the USH2A patients tends to lie between those of the RHO patients and RPGR patients, while the visual field and ERG distributions for the USH2A patients tend to lie to the left of the corresponding distributions for the RHO patients and RPGR patients — similar to what was observed for mean rates of change.
Figure 1.
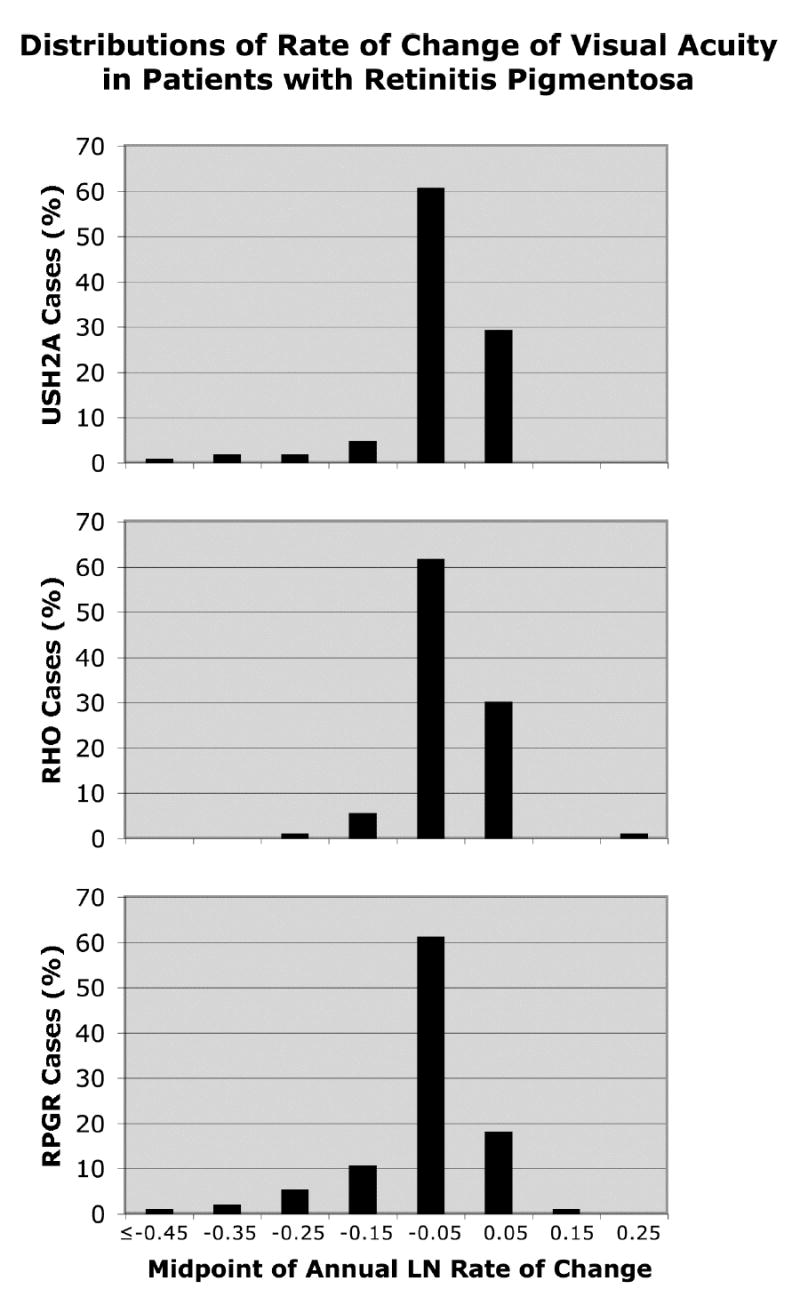
Distributions of rate of change of visual acuity by genotype. Represented are 102 patients with USH2A mutations, 89 patients with RHO mutations, and 93 patients with RPGR mutations.
Figure 3.
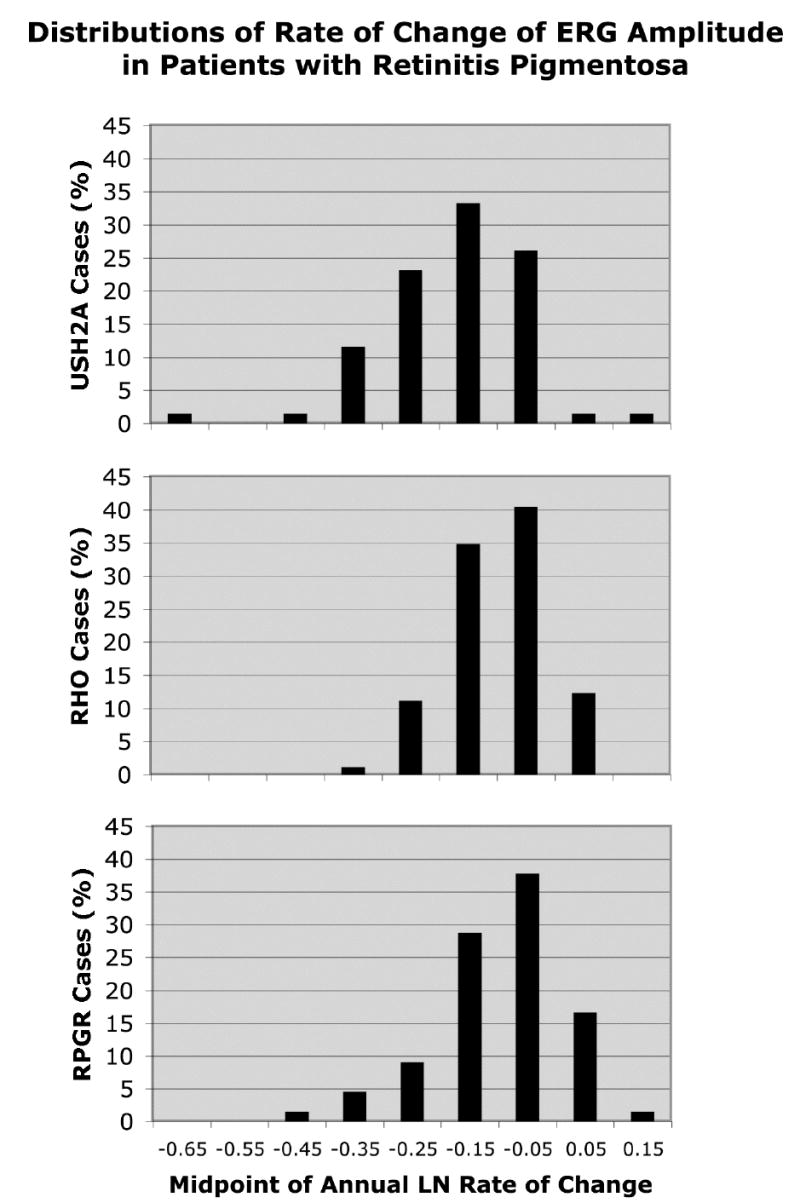
Distributions of rate of change of cone ERG amplitude by genotype. Represented are 69 patients with USH2A mutations, 89 patients with RHO mutations, and 66 patients with RPGR mutations.
Median age to reach legal blindness
We found a significant effect of genotype on the age distribution for legal blindness (p < 0.001). Our patients with USH2A mutations reached legal blindness, based on loss of acuity or field, at a median age of 58 years (Fig 4); this age lies between those previously reported for the patients with RPGR mutations (45 years) and for the patients with RHO mutations (77 years).4 When visual acuity and visual field survival were considered separately, patients with USH2A mutations failed at a median age of 65 years for both measures of ocular function (Fig 4).
Figure 4.
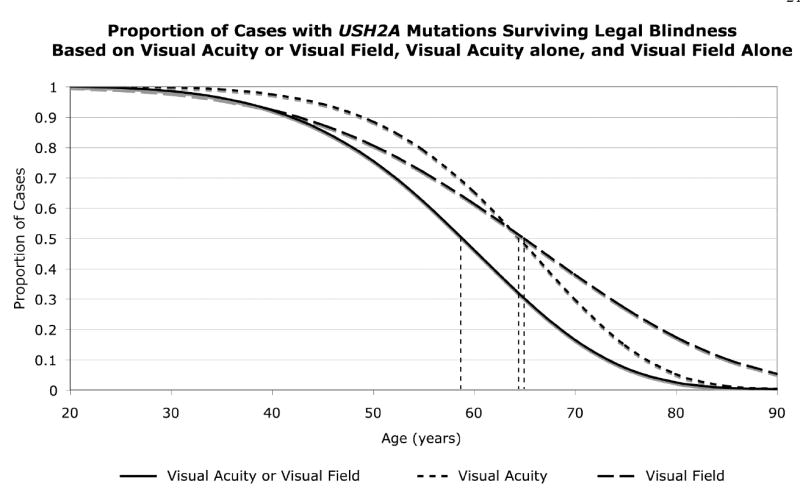
Weibull plot survival analysis for age to legal blindness (i.e., visual acuity ≤ 20/200 or visual field equivalent diameter ≤ 20°), to visual acuity ≤ 20/200, and to visual field equivalent diameter ≤ 20° among patients with USH2A mutations. The vertical dashed lines designate the median ages for legal blindness.
OCTs in patients with the Glu767fs mutation
Figure 5 illustrates tomograms recorded from a normal control and 4 patients with the USH2A Glu767fs mutation. In contrast to the normal tomogram, all 4 patients show a loss of the outer nuclear layer (ONL) outside of the fovea. In addition, the thickness of the outer nuclear layer in the foveal center is smaller for eyes with lower visual acuity in this sample, and the patient with a visual acuity of 20/50 has small off-center cysts.
Figure 5.
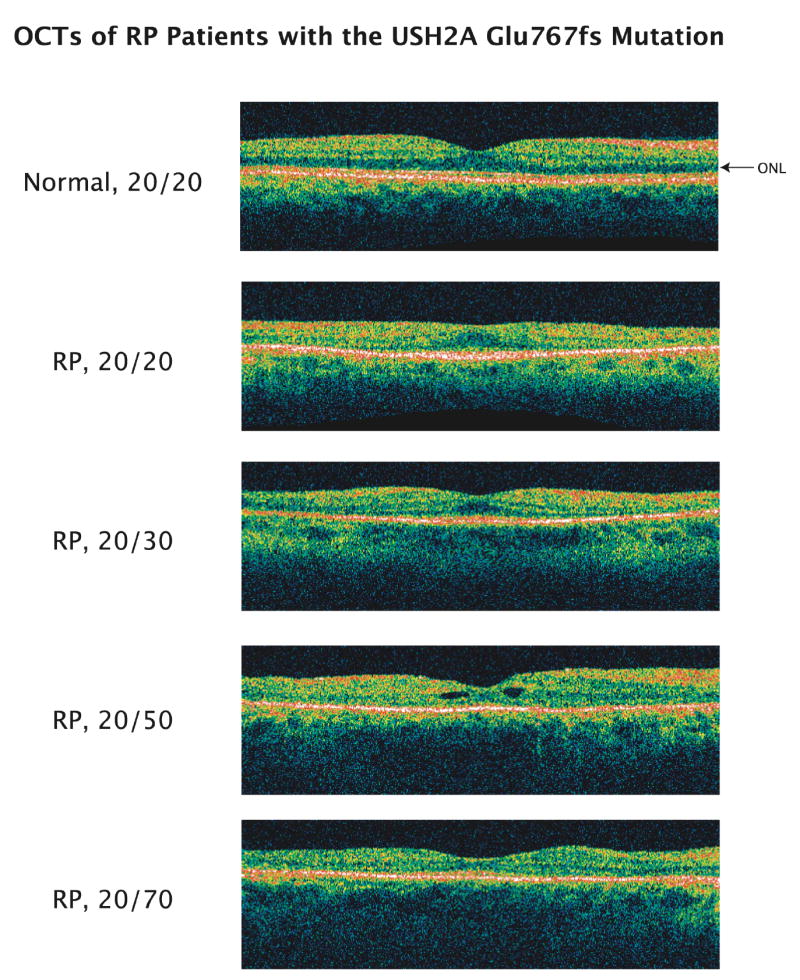
Tomograms from a normal subject and from 4 patients (ages 54, 45, 43, and 52 years from top to bottom) with retinitis pigmentosa (RP) due to the Glu767fs mutation. Images span 6 mm horizontally. The arrow designates the low-reflectance outer nuclear layer (ONL) visible over the full 6 mm in the normal tomogram.
Discussion
The present study shows that patients with retinitis pigmentosa due to USH2A mutations, on average, lost Snellen visual acuity more quickly (i.e., 2.6%/year or ~ 0.6 letter/year) than patients with RHO mutations (i.e., 1.6%/year or ~ 0.4 letter/year) but more slowly than patients with RPGR mutations (i.e., 4.0%/year or ~ 0.9 letter/year). On the other hand, the USH2A patients lost visual field area and full-field cone ERG amplitude, which reflect predominantly extrafoveal function, more rapidly than the other two groups. These differences in mean rates of change could be detected even though patients within a given genotype and for a particular measure of ocular function showed some variation in their rates of change. These results provide a framework for planning clinical trials aimed at stabilizing or slowing the course of this condition.
Two previous studies also evaluated rates of loss of ocular function in patients with USH2A mutations. One of these studies followed Snellen visual acuity in 6 patients and Goldmann visual field in 4 patients but quantified results in “functional” value units that cannot readily be compared to the standard measurements used in the present study.12 The second study followed visual field area in 8 patients with the Glu767fs mutation, censoring areas above their lower limit of normal and data “… that deviated from both preceding and succeeding field areas by 50% or more …” regardless of the intervening time interval.13 These patients had an average rate of decline to the V4e stimulus of 12.1%/year, faster than what we observed in our patients with this mutation (7.7%/year), possibly due to methodological differences. Contrary to our initial hypothesis, we found that patients with nonsyndromic disease (i.e., due to the Cys759Phe mutation) did not have a significantly slower course of disease than patients with retinitis pigmentosa and hearing loss (i.e., due to the Glu767fs mutation).
Our data also revealed that patients with USH2A mutations become legally blind due to loss of visual acuity or visual field at a median age that is older than that of patients with RPGR mutations and younger than that of patients with RHO mutations. Loss of visual acuity and loss of visual field equally contributed to our USH2A patients becoming legally blind. This distinguishes them from RPGR patients, who generally become legally blind due to loss of visual acuity, and RHO patients, who generally become legally blind due to loss of visual field.4
The tomograms recorded from patients with the USH2A Glu767fs mutation showed thinning and loss of the outer nuclear layer with increasing eccentricity consistent with central visual field constriction, thinning of the outer nuclear layer in the center in parallel with decreased visual acuity, and macular cysts in one case. These changes are typical of those that have been described in patients with different forms of retinitis pigmentosa.4,11,14
Figure 2.
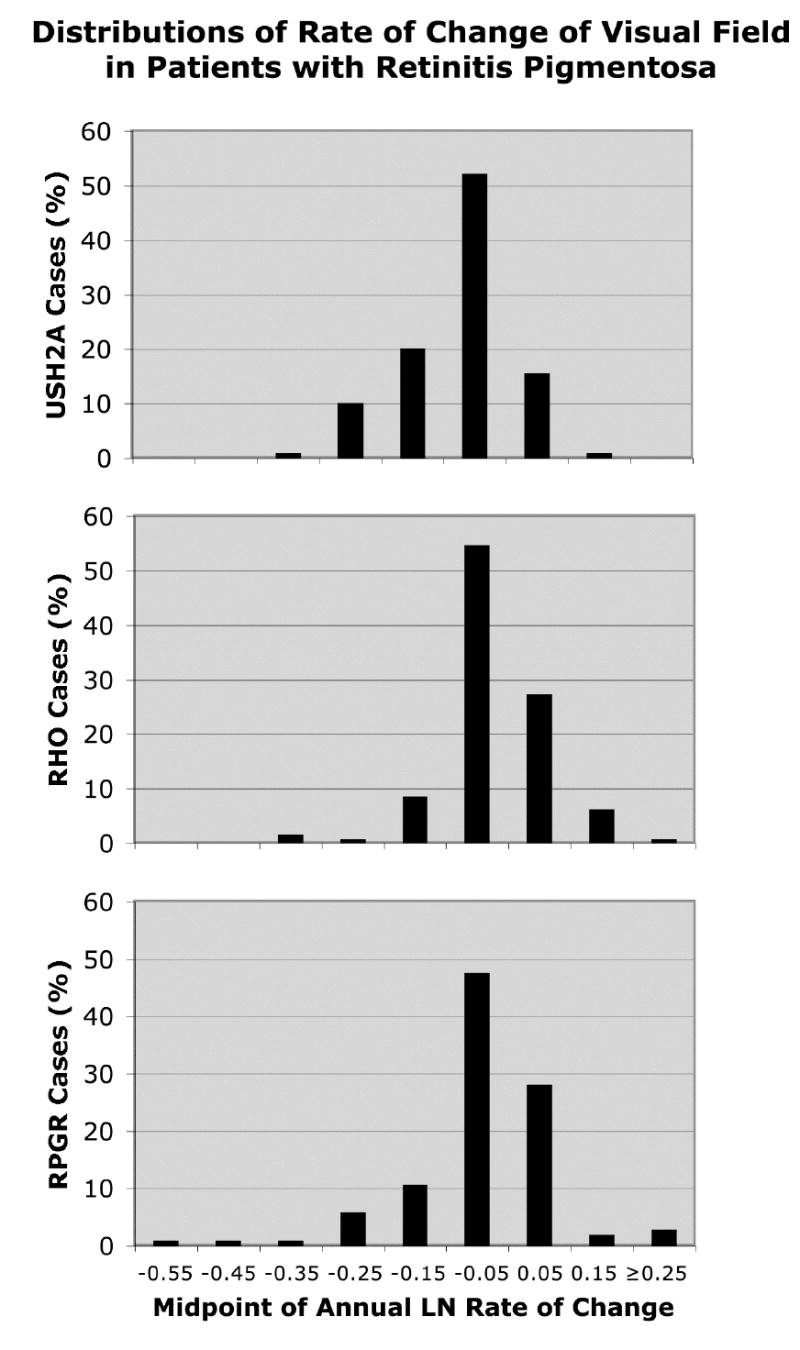
Distributions of rate of change of visual field area by genotype. Represented are 109 patients with USH2A mutations, 128 patients with RHO mutations, and 103 patients with RPGR mutations.
Acknowledgments
Supported by: The National Eye Institute (EY00169, EY08683, and EY14104) and The Foundation Fighting Blindness, Owings Mills, MD.
References
- 1.Dryja TP, McEvoy JA, McGee TL, Berson EL. Novel rhodopsin mutations Gly114Val and Gln184Pro in dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2000;41:3124–3127. [PubMed] [Google Scholar]
- 2.Sharon D, Sandberg MA, Rabe VW, et al. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am J Hum Genet. 2003;73:1131–1146. doi: 10.1086/379379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seyedahmadi BJ, Rivolta C, Keene JA, et al. Comprehensive screening of the USH2A gene in Usher syndrome type II and non-syndromic recessive retinitis pigmentosa. Exp Eye Res. 2004;79:167–173. doi: 10.1016/j.exer.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Sandberg MA, Rosner B, Weigel-DiFranco C, et al. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci. 2007;48:1298–1304. doi: 10.1167/iovs.06-0971. [DOI] [PubMed] [Google Scholar]
- 5.Eudy JD, Weston MD, Yao S, et al. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science. 1998;280:1753–1757. doi: 10.1126/science.280.5370.1753. [DOI] [PubMed] [Google Scholar]
- 6.Weston MD, Eudy JD, Fujita S, et al. Genomic structure and identification of novel mutations in usherin, the gene responsible for Usher syndrome type IIa. Am J Hum Genet. 2000;66:1199–1210. doi: 10.1086/302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Wijk E, Pennings RJ, te Brinke H, et al. Identification of 51 novel exons on the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am J Hum Genet. 2004;74:738–744. doi: 10.1086/383096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer B, Tranebjaerg L, Brox V, et al. A common ancestral origin of the frequent and widespread 2299delG USH2A mutation. Am J Hum Genet. 2001;69:228–234. doi: 10.1086/321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivolta C, Sweklo EA, Berson EL, Dryja TP. Missense mutation in the USH2A gene: association with recessive retinitis pigmentosa without hearing loss. Am J Hum Genet. 2000;66:1975–1978. doi: 10.1086/302926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernal S, Ayuso C, Antinolo G, et al. Mutations in USH2A in Spanish patients with autosomal recessive retinitis pigmentosa: high prevalence and phenotypic variation. J Med Genet. 2003;40:e8. doi: 10.1136/jmg.40.1.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandberg MA, Brockhurst RJ, Gaudio AR, Berson EL. The association between visual acuity and central retinal thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2005;46:3349–3354. doi: 10.1167/iovs.04-1383. [DOI] [PubMed] [Google Scholar]
- 12.Pennings RJE, Huygen PLM, Orten DJ, et al. Evaluation of visual impairment in Usher syndrome 1b and Usher syndrome 2a. Acta Ophthalmol Scand. 2004;82:131–139. doi: 10.1111/j.1600-0420.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 13.Fishman GA, Bozbeyoglu S, Massof RW, Kimberling W. Natural course of visual field loss in patients with Type 2 Usher syndrome. Retina. 2007;27:601–608. doi: 10.1097/01.iae.0000246675.88911.2c. [DOI] [PubMed] [Google Scholar]
- 14.Witkin AJ, Ko TH, Fujimoto JG, et al. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol. 2006;142:945–952. doi: 10.1016/j.ajo.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


