Abstract
Many neuromuscular conditions are characterized by an exaggerated exercise-induced fatigue response that is disproportionate to activity level. This fatigue does not necessarily correlate with elevated central or peripheral fatigue in patients1, and some patients experience severe fatigue without any demonstrable somatic disease2. Except in myopathies that are due to specific metabolic defects, the mechanism underlying this type of fatigue remains unknown2. With no treatment available, this form of inactivity is a major determinant of disability3. Here we show, using mouse models, that this exaggerated fatigue response is distinct from a loss in specific force production by muscle, and that sarcolemma-localized nNOS signaling in skeletal muscle is required to maintain activity after mild exercise. Of significance, we show that nNOS-null mice do not have muscle pathology and have no loss of muscle specific-force after exercise, but do display this exaggerated fatigue response to mild exercise. In mouse models of nNOS mislocalization from the sarcolemma, prolonged inactivity was only relieved by pharmacologically enhancing the cGMP signal that results from muscle nNOS activation during the nitric oxide signaling response to mild exercise. Our findings suggest that the mechanism underlying the exaggerated fatigue response to mild exercise is a lack of contraction-induced signaling from sarcolemma-localized nNOS, which reduces cGMP-mediated vasomodulation in the vessels that supply active muscle after mild exercise. Notably, sarcolemmal nNOS was reduced in patient biopsies from a large number of distinct myopathies, suggesting a common mechanism of fatigue. Our results suggest that patients with an exaggerated fatigue response to mild exercise would show clinical improvement in response to treatment strategies aimed at improving exercise-induced signaling.
To understand the molecular basis of the exercise-induced fatigue response, we studied genetically-defined mouse models. We designed an integrative in vivo assay to test conscious mice, subjecting the mice to brief low-speed treadmill exercise followed by testing in an open-field activity chamber (see methods). We first assessed two dystrophic mouse lines – mdx (model for Duchenne Muscular Dystrophy (DMD))4 and Sgca-null (model for limb-girdle muscular dystrophy type 2D that is deficient for α-sarcoglycan (Sgca))5. In the absence of previous exercise, activity in these mice was indistinguishable from that of wild-type mice (Supplementary videos S1a-d, Fig. 1a-b). After a single trial of mild exercise, significant differences were observed (Supplementary videos S2a-d, Fig. 1a-b) – the mdx and Sgca-null mice showed significant reduction in vertical activity.
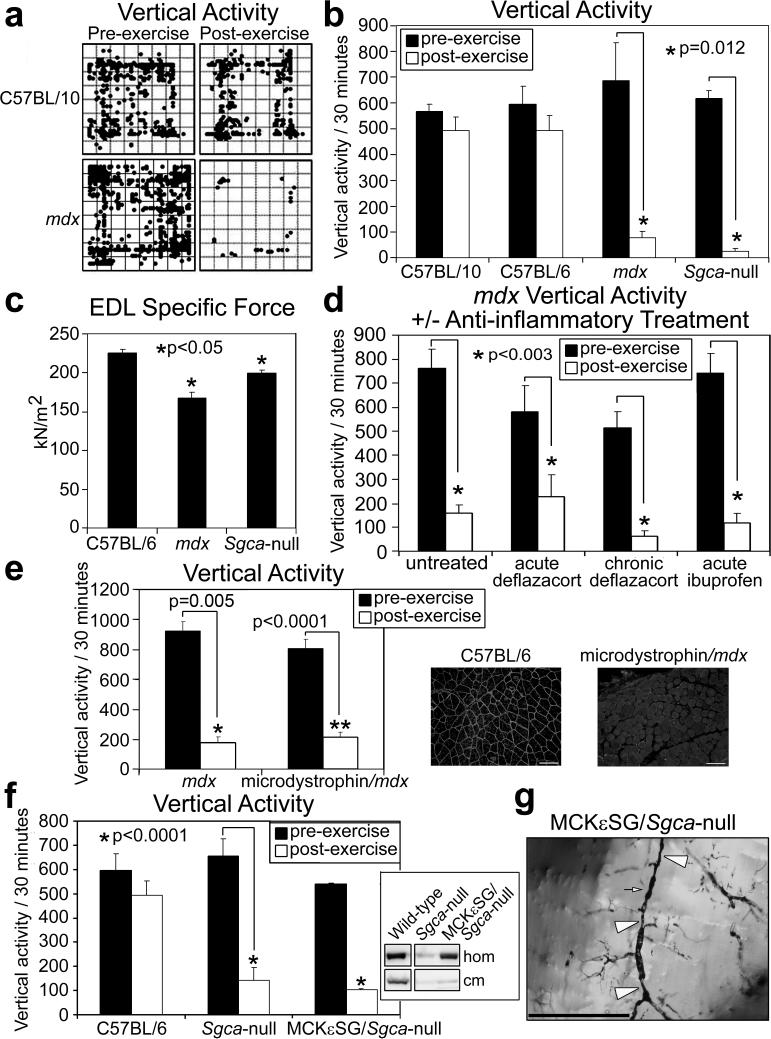
Figure 1. Loss of sarcolemma-localized nNOS leads to skeletal muscle vascular narrowings, reduced capillary perfusion, and an exaggerated fatigue response after mild exercise in dystrophic and non-dystrophic mouse models.
a, Representative C57BL/10 and mdx mice pre- and post-exercise zone map vertical activity tracings. b, Quantified vertical activity pre- and post-exercise for C57BL/10, C57BL/6, mdx and Sgca-null mouse strains (n=6 for each strain). c, EDL muscle specific force measurements from C57BL/6 (n=6), mdx (n=4), and Sgca-null (n=4) mice. d, Pre- and post-exercise vertical activity in untreated (n=7) and anti-inflammatory treated mdx mice, acutely (n=4) or chronically (n=4) with deflazcort or acutely with ibuprofen (n=5). Asterisks indicate statistical significance. e, Quantified pre and post-exercise vertical activity for Microdystrophin/mdx mice (n=6) and their mdx littermates (n=4). Insets show representative immunofluorescence images of nNOS detection in the gastrocnemius muscles from C57BL/6 and microdystrophin/mdx mice. f, Quantified pre and post-exercise vertical activity for MCKεSG/Sgca-null mice (n=6) and their Sgca-null littermates (n=6). Inset shows immunoblot detection of total nNOS from homogenates (hom), and crude skeletal muscle membranes (cm). g, Representative Microfil® image of skeletal muscle vessels of MCKεSG/Sgca-null mice post-exercise – large arrow heads mark extended areas of vascular narrowing and small arrows mark shorter stretches of radial vascular narrowing.
The drop in the vertical activity among mdx and Sgca-null mice did not correlate with differences in EDL-specific force measurements relative to those taken in C57BL/6 mice before exercise (Fig. 1c). Moreover, Sgca-null mice do not develop brain, heart nor vascular pathology6, and have muscle-force values similar to those of control mice7. Therefore, neither cardiac deficiency nor an inability to produce force was the cause of the post-exercise inactivity in the Sgca-null mice. Since inflammation is a feature of dystrophinopathy4, chronic fatigue is associated with muscle pain, and chronic pain is associated with fatigue8, we treated mdx mice with either deflazacort or ibuprofen. However, neither treatment resulted in improved post-exercise activity (Fig. 1d) suggesting that the inactivity that occurs immediately after mild exercise in mdx mice was not due to inflammation or pain. Overall, the results of our exercise-activity assay implied that the exaggerated fatigue response in these mice was not attributable to cardiac deficiency, lack of muscle force, inflammation, or pain.
To test if the exercise-induced inactivity in the mdx and Sgca-null mice was due to the genetically determined structural defect in muscle, we assayed two mouse models in which the muscle pathology related to the specific dystrophin glycoprotein complex (DGC) defect is rescued – Microdystrophin/mdx and MCKεSG/Sgca-null. In microdystrophin/mdx mice (model for mild Becker MD9 – the DGC has a mutated but functional dystrophin), microdystrophin is expressed in mdx mouse muscle. In the MCKεSG/Sgca-null mice, ε-sarcoglycan is expressed in mouse muscle that is deficient for Sgca (Supplementary Fig. S1). Neither rescue strain showed pathological signs of muscular dystrophy, and the skeletal muscle DGC of both was recovered at the biochemical, structural, and functional levels (9, 10 and Supplemental Fig. S1, S2).
Despite having a structurally intact skeletal muscle DGC, microdystrophin/mdx mice experience a substantial drop in activity after mild exercise, like their mdx littermates (Fig. 1e). Since Becker MD patients show profound fatigue after light exertion11, and loss of sarcolemma-localized nNOS serves as a diagnostic indicator of some forms of Becker MD12, a possible reason for the post-exercise inactivity is a loss of sarcolemma-localized nNOS. To test this possibility, we probed for nNOS localization in microdystrophin/mdx skeletal muscle, and found that the DGC generated in this rescue strain failed to recruit nNOS to the sarcolemma (Fig. 1e, inset). These data are in agreement with recent reports on microdystrophin expression in dystrophin-deficient mouse models13. Moreover, the data suggest that exercise-induce inactivity in the microdystrophin/mdx mice is not directly caused by a structurally defective muscle DGC, and that loss of sarcolemmal nNOS does not negatively affect muscle contractility. Thus, sarcolemmal nNOS appears to act at the level of post-exercise activity.
In contrast to the microdystrophin/mdx mice, MCKεSG/Sgca-null mice have structurally intact DGC in the brain and the vasculature, but express ε-sarcoglycan instead of Sgca in the DGC of muscle. Our exercise-activity assay showed that post-exercise activity in the MCKεSG/Sgca-null mice was substantially decreased relative to that in C57BL/6 mice, but similar to that in Sgca-null and mdx mice (Fig. 1b, 1f). Since the microdystrophin-containing DGC failed to recruit nNOS, we speculated that the MCKεSG/Sgca-null mice would also fail to localize nNOS to the sarcolemma. Indeed, although total nNOS levels in muscle homogenates from MCKεSG/Sgca-null mice were similar to wild-type levels, nNOS from the rescue model failed to co-purify with the ε-sarcoglycan-containing DGC in the membrane preparation (Fig. 1f, inset). Together, these results are compatible with the exaggerated fatigue response not being directly related to a structurally defective muscle DGC or to muscle weakness, but rather to a failure in the sarcolemmal localization of nNOS.
Since sarcolemma localized nNOS is crucial for maintaining vasomodulation to contracting muscles14, we tested if communication from skeletal muscle to the local blood supply is deficient after mild exercise by perfusing MCKεSG/Sgca-null mouse arteries pre- or post-exercise with Microfil® and examined the skeletal muscle vasculature (Fig. 1g). We identified vascular narrowings of varying lengths along the arteries that feed the skeletal muscles only in the post-exercise samples and also noted the lack of perfusion of capillaries. The mdx and microdystrophin/mdx mice likewise showed skeletal muscle vascular narrowings only post-exercise and a lack of perfusion of capillaries (Supplementary Fig. S3c and data not shown). This phenotype is consistent with inefficient contraction-induced muscle nNOS signaling to local blood vessels. Overall, these data imply that loss of sarcolemma-localized nNOS causes deficient exercise-induced vasomodulation in skeletal muscle, and that these lead to prolonged inactivity post-exercise.
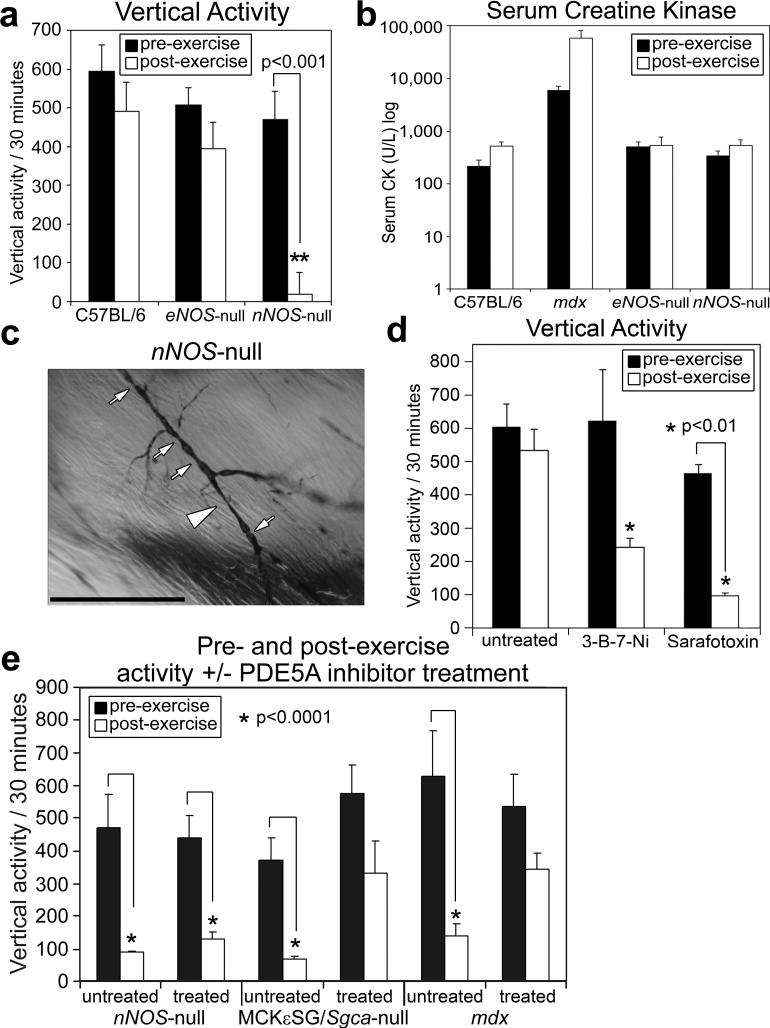
To directly examine the contribution of NO generated by eNOS or nNOS to the exaggerated fatigue response, we tested both nNOS- and eNOS-null mice in our exercise-activity assay. Mice deficient for nNOS express normal levels of the DGC components at the sarcolemma and have histologically normal muscle15-17. Reports suggest that both mouse strains have defective vasoregulation18, 19; however, mdx and nNOS-null mice have a normal α-adrenergic vasoconstrictive response to exercise20. Vertical pre-exercise activities were similar in eNOS-null, nNOS-null, and C57BL/6 mice, suggesting that the loss of either NOS does not affect mouse activity (Fig. 2a). Post-exercise, however, nNOS-null vertical activity dropped significantly (Fig. 2a). Serum CK levels pre- and post-exercise for each of the NOS-null mice were similar to those in C57BL/6 mice and low compared to mdx mice (Fig. 2b), and there were no signs of muscle pathology in nNOS-null quadriceps muscle sections (Supplementary Fig. S4b), suggesting that muscle damage and necrosis were not the causes of the post-exercise inactivity. We then tested if post-exercise muscle contractility affected the ability of C57BL/6 and nNOS-null skeletal muscle to produce force after mild exercise. We found that the specific force of EDL muscles after exercise was not significantly affected in nNOS-null muscle compared to C57BL/6 muscle (Supplementary Fig. S4c). Since lack of muscle contractility was not causing the inactivity in the nNOS-null mice post-exercise, we checked whether NOS-null mice had post-exercise skeletal muscle vascular narrowings and lack of capillary perfusion similar to those in the dystrophic and rescue mice. Microfil® perfusion of arteries of NOS-null mouse arteries pre- and post-exercise revealed the lack of capillary perfusion and also the presence of vascular narrowings only in post-exercise nNOS-null skeletal muscle (Fig. 2c). We also found that treating wild-type mice with either the nNOS-specific inhibitor 3-bromo-7-nitroindazole or the vasoconstrictor sarafotoxin 6c caused post-exercise inactivity (Fig. 2d). These findings suggest that a deficiency of sarcolemma-localized nNOS causes exercise-induced narrowing of the vasculature that feeds active muscles after exercise, thereby promoting prolonged inactivity after mild exercise.
Figure 2. Enhancing the cGMP signal resulting from muscle nNOS activation decreases the exaggerated fatigue response to mild exercise.
a, Comparison of vertical activity post-exercise among C57BL/6, eNOS- and nNOS-null mice (n=6 for each). b, Serum creatine kinase levels pre- and post-exercise in C57BL/6 (n=6), eNOS-null (n=4), and nNOS-null (n=6) mice, compared to mdx (n=6). c, Representative Microfil® image of nNOS-null quadriceps skeletal muscle arteries post-exercise – large arrow heads mark extended area of vascular narrowing and small arrows mark shorter areas of radial vascular narrowing. d, Pre- and post-exercise vertical activities in untreated wild-type (C57BL/6 and C57BL/10) mice (n=4), compared to 3-B-7-Ni treated wild-type mice (n=3), and to sarafotoxin treated wild-type mice (n=4). e, Quantified pre- and post-exercise activity +/−PDE5A inhibitor treatment, in nNOS-null (n=4), MCKεSG/Sgca-null (n=4), and mdx mice (n=6). Pre- and post-exercise vertical activity error bars are s.e.m., scale bar = 100 μm, and asterisks indicate statistical significance.
To test if the vascular effect on post-exercise activity was from NO or downstream of the NO signal, we bypassed sarcolemmal nNOS signaling for reducing vasoconstriction by treating mdx mice with a panel of pharmacological agents that promote vasodilation; we found that the exaggerated fatigue response was alleviated only by phosphodiesterase (PDE) 5A inhibitor treatment (Supplementary Fig. S6), suggesting that the fatigue we see depends on cGMP, which acts downstream of NO production. Interestingly, PDE activity in mdx mice is 2−6x higher than in C57BL/10 mice21, consistent with the elevated PDE activity in human muscular disorders18, 21, 22. We treated nNOS-null, MCKεSG/Sgca-null, and mdx mice with PDE5A inhibitors and tested them in our exercise-activity assay and found that the treated MCKεSG/Sgca-null and mdx mice showed an increase in post-exercise activity (Fig. 2e and Supplemental Fig. S7a-d). Since PDE5A inhibition had no effect on activity before exercise, our results suggest that PDE5A inhibition is alleviating the exaggerated fatigue response by enhancing the cGMP signal produced by contraction-induced nNOS stimulation. Although downstream effectors of cGMP are numerous and divergent23, the half-life of cGMP can be affected by the activity of PDE5A. Our data suggest that the elevated PDE activity in mdx mouse extracts could be PDE5A activity and that PDE activity could also be elevated in the rescue mouse models we tested.
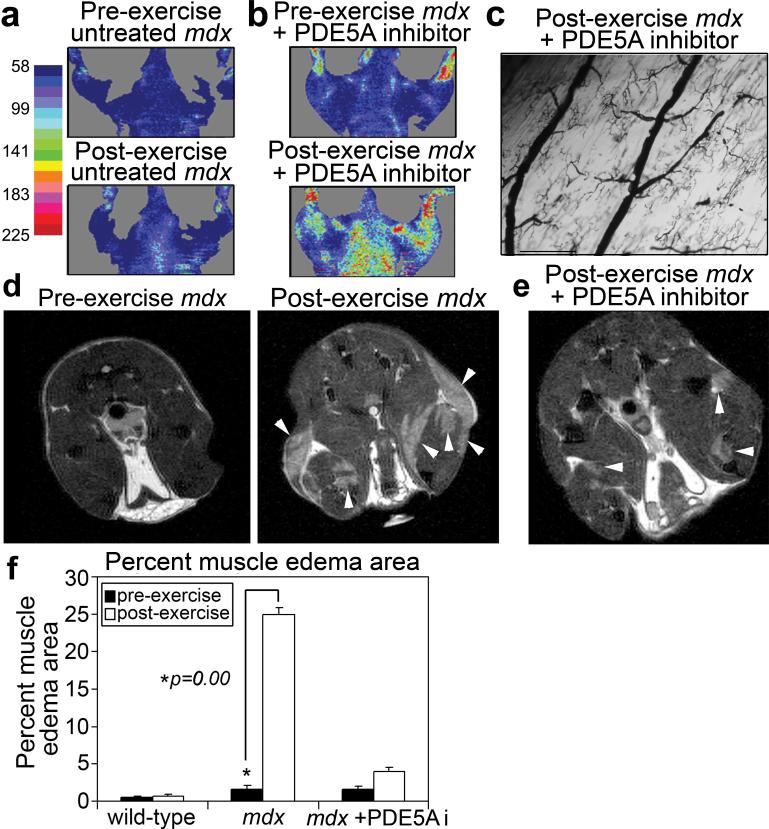
Our data suggest that the local resistance of arterioles that perfuse sarcolemmal nNOS-deficient muscles increases during exercise and that the lack of activity after mild exercise will lead to muscle edema. We examined blood flow pre- and post-exercise with Laser Doppler imaging and found that blood flow in mdx mice failed to increase as it did in C57BL/6 mice (Fig. 3a and Supplemental Fig. S7a), but that treatment of mdx mice with a PDE5A inhibitor alleviated this defect (Fig. 3b) and increased muscle capillary perfusion (Fig. 3c). Given that insufficient relief of local vasoconstriction in active muscles can lead to muscle edema24, and that boys afflicted with DMD show muscle edema25, we looked for changes in water compartmentalization and dynamics in the hind-leg muscles of nNOS-null, C57BL/10 and mdx mice before and after exercise using spin-spin relaxation time (T2)-magnetic resonance imaging (MRI). The nNOS-null mice did not have muscle damage or loss of contractility after exercise (Fig. 2b and Supplementary Fig. S4b-c), nor did they have muscle edema (Supplemental Fig. S8a), suggesting that their lack of muscle damage prevents water accumulation in the tissue. Similarly, C57BL/10 mice showed little to no hind-leg muscle edema post-exercise (0.70% +/− 0.50) (Fig. 3f and Supplementary Fig. S8b). However, hind-leg muscles of mdx mice consistently showed significant changes in tissue hydration post-exercise (25.0% +/− 2.45) (Fig. 3d, f) that were indicative of exercise-induced muscle edema. The water accumulation observed in the mdx muscles is likely due to a combination of the increased local resistance in the arterioles that feed the active leg muscles and of muscle fiber fragility and damage. We also consistently found that PDE5A inhibitor treatment significantly reduced exercise-induced muscle edema in mdx mice (3.99% +/− 0.82) (Fig. 3e, f). Overall, our data imply that PDE5A inhibitor treatment can relieve the post-exercise inactivity by normalizing PDE activity, thereby allowing the available NO derived from muscle nNOS to signal for cGMP-dependent vasodilation in active muscle; PDE5A inhibitor treatment reduces muscle damage in mdx mice by improving modulation of vascular activity in active muscle, thus preventing muscle edema from exacerbating the muscle damage that occurs during the contraction of dystrophic muscle.
Figure 3. PDE5A inhibitor treatment improves exercised-induced vasomodulation and reduces exercise-induced edema in mdx mice.
a, Representative images of coronal Laser Doppler analysis of blood flow from mdx mice pre- and post-exercise (n=3), and b, mdx mice pre- and post-exercise + PDE5A inhibitor treatment (n=3). c, Representative Microfil® image of mdx + PDE5A inhibitor treatment quadriceps skeletal muscle arteries post-exercise (n=3, Scale bar = 100 μm). d-e, Representative MRI axial views of: d, mdx hind-limb muscles pre- and post-exercise (n=5) and e, hind-limb muscles post-exercise of mdx treated with PDE5A inhibitor before exercise (n=5). White arrowheads mark areas of increased water compartmentalization. f, Percent muscle edema area preand post-exercise and +/− PDE5A inhibitor treatment in mdx mice compared to that of wild-type. (wild-type and mdx n=3, mdx + PDE5A inhibitor n=5, error bars are s.e.m.)
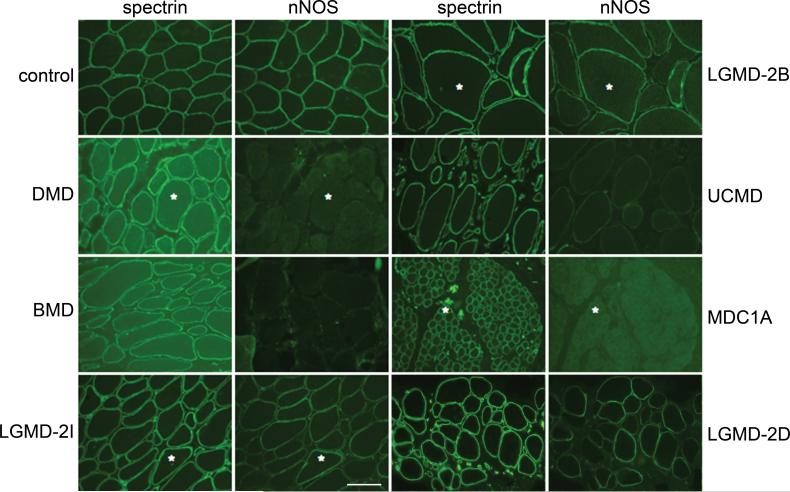
Since more than 60% of all patients with neuromuscular disease suffer from severe fatigue2, we tested for nNOS localization to the sarcolemma in patient biopsies representing different myopathic disorders (Fig. 4, Supplementary Fig. S9 and Table 1). In most myopathic biopsies assessed, sarcolemma-localized nNOS was either reduced or not detected, implying that many myopathic disorders may share a mechanism that results in severe exercise-induced fatigue. Although increased fatigability inevitably occurs in patients with muscle weakness1, our mouse data imply that the exercise-induced inactivity is distinct from muscle weakness and that loss of sarcolemma-localized nNOS leads to an exaggerated fatigue response to mild exercise.
Figure 4. nNOS is reduced in human muscle diseases.
Representative immunofluorescent staining in various human muscle diseases: in primary dystrophinopathies, Duchenne and Becker muscular dystrophy (DMD and BMD, respectively); in several forms of limb-girdle muscular dystrophy (LGMD); in two congenital muscular dystrophies (CMD) caused by mutations in extracellular matrix proteins [Ullrich CMD (UCMD), collagen VI and merosin-deficient CMD (MDC1A), laminin-2]. Asterisks mark the same muscle fibers in some of the adjacent panels. (Scale bar = 100 μm)
In conclusion, our mouse data show that reduced or mislocalized skeletal muscle nNOS exacerbates the fatigue experienced after mild exercise because the normal contraction-induced cGMP-dependent attenuation of local vasoconstriction fails to occur, and that this failure causes vascular narrowing in muscles after exercise. In addition, our mdx mouse data suggest that due to nNOS mislocalization and increased PDE activity10, 18, 21, signaling for increased vasodilation to active muscle is deficient, causing muscle edema. This, in turn, contributes to increased muscle damage as well as profound post-exercise debility. Although the exact mechanism that leads to the inactivity after mild exercise has not been reduced to one single beginning and end pathway, our data suggest that contraction-induced cGMP-dependent attenuation of local vasoconstriction is pivotal in this mechanism. These findings could lead to a better understanding of muscle fatigue under other physiological conditions in which muscle nNOS expression, localization, or activity is affected.
METHODS SUMMARY
Full Methods and Additional Methods are available in Supplementary Information.
Mouse models
Animal care and procedures were approved and performed in accordance with the standards set forth by the National Institutes of Health and the University of Iowa Animal Care and Use Committee.
Treadmill exercise and activity monitoring
Animals were mildly exercised using an adjustable variable speed belt treadmill from AccuPacer. Activity based on ambulatory behavior was assessed in an open-field test.
Supplementary Material
Acknowledgements
We thank Drs. M. Anderson and M. Henry for comments and M.M. Kilburg, K. Uppal, B.J. Steinmann, and S. Watkins and members of the Campbell laboratory for scientific contributions. This work was supported in part by a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant. Y.M.K. was supported by grants from the University of Iowa Cardiovascular Interdisciplinary Research/NRSA Fellowship, from an individual NRSA Fellowship from the NIAMS, from NIH, and from a Senator Paul D. Wellstone Fellowship. E.P.R was supported by an MDA Development Grant. R.M.W. was supported by NIH. K.P.C. is an investigator of the Howard Hughes Medical Institute. The authors declare no conflicts of interests.
Footnotes
Supplementary information and additional methods accompanies this paper on www.nature.com/nature.
REFERENCES
- 1.Schillings ML, et al. Experienced and physiological fatigue in neuromuscular disorders. Clin Neurophysiol. 2007;118:292–300. doi: 10.1016/j.clinph.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Zwarts MJ, Bleijenberg G, van Engelen BG. Clinical neurophysiology of fatigue. Clin Neurophysiol. 2007 doi: 10.1016/j.clinph.2007.09.126. doi:10.1016/j.clinph.2007.09.126. [DOI] [PubMed] [Google Scholar]
- 3.Kalkman JS, Schillings ML, Zwarts MJ, van Engelen BG, Bleijenberg G. The development of a model of fatigue in neuromuscular disorders: A longitudinal study. J Psychosom Res. 2007;62:571–9. doi: 10.1016/j.jpsychores.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007;39:469–77. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Duclos F, et al. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J Cell Biol. 1998;142:1461–71. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozawa E, Mizuno Y, Hagiwara Y, Sasaoka T, Yoshida M. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve. 2005;32:563–76. doi: 10.1002/mus.20349. [DOI] [PubMed] [Google Scholar]
- 7.Consolino CM, et al. Muscles of mice deficient in alpha-sarcoglycan maintain large masses and near control force values throughout the life span. Physiol Genomics. 2005;22:244–56. doi: 10.1152/physiolgenomics.00311.2004. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain. 2007;8:692–9. doi: 10.1016/j.jpain.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harper SQ, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–61. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 10.Imamura M, Mochizuki Y, Engvall E, Takeda S. i. {varepsilon}-Sarcoglycan compensates for lack of {alpha}-sarcoglycan in a mouse model of limb-girdle muscular dystrophy. Hum. Mol. Genet. 2005;14:775–783. doi: 10.1093/hmg/ddi072. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BA, Mastaglia FL. Exercise therapy in patients with myopathy. Curr Opin Neurol. 2000;13:547–52. doi: 10.1097/00019052-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Torelli S, et al. Absence of neuronal nitric oxide synthase (nNOS) as a pathological marker for the diagnosis of Becker muscular dystrophy with rod domain deletions. Neuropathol Appl Neurobiol. 2004;30:540–5. doi: 10.1111/j.1365-2990.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 13.Judge LM, Haraguchiln M, Chamberlain JS. Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex. J Cell Sci. 2006;119:1537–46. doi: 10.1242/jcs.02857. [DOI] [PubMed] [Google Scholar]
- 14.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal Nitric oxide synthase. Circ Res. 2003;92:554–60. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- 15.Chao DS, Silvagno F, Bredt DS. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J Neurochem. 1998;71:784–9. doi: 10.1046/j.1471-4159.1998.71020784.x. [DOI] [PubMed] [Google Scholar]
- 16.Crosbie RH, et al. mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet. 1998;7:823–9. doi: 10.1093/hmg/7.5.823. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki N, et al. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest. 2007;117:2468–2476. doi: 10.1172/JCI30654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asai A, et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang PL, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GD, et al. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci U S A. 1998;95:15090–5. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom TJ. Age-related alterations in cyclic nucleotide phosphodiesterase activity in dystrophic mouse leg muscle. Can J Physiol Pharmacol. 2005;83:1055–60. doi: 10.1139/y05-085. [DOI] [PubMed] [Google Scholar]
- 22.Bloom TJ. Cyclic nucleotide phosphodiesterase isozymes expressed in mouse skeletal muscle. Can J Physiol Pharmacol. 2002;80:1132–5. doi: 10.1139/y02-149. [DOI] [PubMed] [Google Scholar]
- 23.Kass DA, Champion HC, Beavo JA. Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res. 2007;101:1084–95. doi: 10.1161/CIRCRESAHA.107.162511. [DOI] [PubMed] [Google Scholar]
- 24.Persson J, Ekelund U, Grande PO. Endogenous nitric oxide reduces microvascular permeability and tissue oedema during exercise in cat skeletal muscle. J Vasc Res. 2003;40:538–46. doi: 10.1159/000075677. [DOI] [PubMed] [Google Scholar]
- 25.Marden FA, Connolly AM, Siegel MJ, Rubin DA. Compositional analysis of muscle in boys with Duchenne muscular dystrophy using MR imaging. Skeletal Radiol. 2005;34:140–8. doi: 10.1007/s00256-004-0825-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.