Abstract
Social cognition in humans is distinguished by psychological processes that allow us to make inferences about what is going on inside other people—their intentions, feelings, and thoughts. Some of these processes likely account for aspects of human social behavior that are unique, such as our culture and civilization. Most schemes divide social information processing into those processes that are relatively automatic and driven by the stimuli, versus those that are more deliberative and controlled, and sensitive to context and strategy. These distinctions are reflected in the neural structures that underlie social cognition, where there is a recent wealth of data primarily from functional neuroimaging. Here I provide a broad survey of the key abilities, processes, and ways in which to relate these to data from cognitive neuroscience.
Keywords: social cognition, social neuroscience, theory of mind, simulation, empathy, amygdala, prefrontal cortex, modularity
Knowledge of Minds
The basic fact is thus that human beings are able to pool their cognitive resources in ways that other species are not … made possible by a single very special form of social cognition, namely, the ability of individual organisms to understand conspecifics as beings like themselves who have intentional and mental lives like their own. (Tomasello 1999)
Comparative Studies
We are an essentially social species; no component of our civilization would be possible without large-scale collective behavior. Yet much of our social behavior arises from neurobiological and psychological mechanisms shared with other mammalian species, raising questions about why we are different. Part of this difference may arise from knowledge of our own minds and those of others, a type of knowledge different from that about the shared nonsocial environment, and in degree if not in kind inaccessible and inconceivable to nonhuman animals.
There are three broad domains of knowledge that, taken together, seem to exhaust what it is that we can know or conceive of knowing. The first is the simplest to describe—it is knowledge of the nonsocial environment, the world we share with others. The common-sense view is that this domain of knowledge is shared, public, and hence objective in that sense. How we come to acquire this knowledge is also no mystery—through our senses and perception of the world (although the acquisition of such knowledge already depends on learning, selection, and categorization mechanisms that are in part innate). Although the kinds of inferences that we make about the world are certainly complex, it seems that much of this domain of knowledge is shared with other animals. Like us, mice, cats, dogs, and monkeys know about objects in the world, the properties they possess, and the events they transact; they know something about which objects are good and which are bad, and they direct their behavior accordingly.
The second and third domains of knowledge are more mysterious, and it is unclear to what extent, if at all, other animals have access to them. These are knowledge of other minds, and knowledge of our own mind. Although many biologists who study social behavior in animals treat their processing of social information as an issue in perception that is just a special instance of the first category discussed above, some, especially those working with primates, focus on knowledge of one's own and others' minds. Workhorse tasks have been devised to assess the abilities in question: deception as a test for knowledge of other minds, and mirror self-recognition as a test for self-knowledge.
Knowledge of the Minds of Others
One way of knowing about the social world, of course, is through the same processes by which we know about the nonsocial world. There is overwhelming evidence that many animals are able to use social cues in sophisticated ways, and primates especially are able to track kinship and social rank over time (Cheney & Seyfarth 1990, Silk 2005)—abilities that make substantial demands on several cognitive domains, such as episodic memory. But it is also possible that the processes used for such social knowledge differ in important ways from those engaged in nonsocial knowledge: Many of these abilities look as if the animals were inferring mental states by them. For instance, dogs, unlike wolves, when faced with a problem that they cannot solve, know to look back to their owner in order to see what that person recommends they do (Miklosi et al. 2003). Thus, they know that the human “knows” something that can help them if they need additional information. Monkeys are able to distinguish human actions that are intentional: They fail to show any preference to eat from a bowl of food to which a human points with an elbow (because pointing with one's elbow doesn't make sense normally), but they do show a preference to eat from the bowl if a human points with an elbow while holding something else with both hands (because in that case the hands are not free to point and using the elbow makes sense) (Wood et al. 2007). However one wants to interpret these abilities, there is still doubt that they are sufficient to attribute a concept of “mind” or “subjective” or “consciousness” to animals (although, of course, higher animals do have minds together with subjective conscious experiences; it is just that they may not know that they do).
There is a large literature on experiments in our closest living relative, the chimpanzee, to attempt to demonstrate that they really do have a concept of other minds (although it is granted that the chimp's concept of mind would not be the same as the human's). The question was first posed explicitly in a famous article in the 1970s (Premack & Woodruff 1978) and was followed by a commentary in which philosopher Daniel Dennett recommended what has since become a common experimental strategy: To show that an animal can conceive of minds (has a “theory of mind”), one must demonstrate a concept of false belief, which has been operationalized in animals as the ability to deceive. The reasoning here is that one must decouple the state of someone's mind (e.g., what they believe) from the state of the world (e.g., what they perceive). There are fairly detailed experiments of chimpanzee deception (Tomasello et al. 2003), and it certainly appears to be the case that chimpanzees are sensitive to what other chimps know and are able behaviorally to deceive them, although the interpretation of these findings remains debated (Povinelli & Vonk 2003). Daniel Povinelli has proposed an interesting experiment that joins knowledge of other minds with knowledge of one's own mind and that could be more decisive if it worked. Suppose we construct a helmet one can wear, that looks from the outside like a completely opaque bucket. Now, without any prior interaction with a person wearing one of these helmets, the chimp is given a red and a blue helmet to wear itself. It turns out that only the red helmet has a little monitor inside that is hooked up to a video camera, such that one can see what is in front of the helmet when one wears it. If the chimp, after having experienced “seeing” itself while wearing the red helmet, an experience completely novel and hence not subject to any prior associations, now begs for food from people wearing the red but not the blue helmet, this could count as unambiguous evidence that chimps can attribute mental states to others, with extrapolation from their own and unique conscious experience as the sole source of the inference. So far, there is no evidence that chimps can pass this test (Penn & Povinelli 2007), although this negative finding can be criticized on a number of grounds including the limited number of animals that have been tested on it and its highly artificial nature (compared to what chimps might be expected to encounter in nature). In fact, it has been pointed out that both the human samples often tested in such experiments (white, middle-class, Western humans) and the chimpanzee samples (captive chimpanzees) are highly atypical (Boesch 2007), making generalizations drawn from them unclear.
Knowledge of One's Own Mind
Demonstrating knowledge of one's own mind in animals is at an even more problematic stage. A classic test, mirror self-recognition, seems adequate to show recognition of one's own body but insufficient to show knowledge of one's own mind. Although it used to be thought that only great apes could recognize their own body in a mirror (Gallup 1970), such discrimination has now been shown for monkeys (de Waal et al. 2005), dolphins (Reiss & Marino 2001), and elephants (Plotnik et al. 2006). These tests, for their complete assessment, rely on the ability of the animal to behave on the basis of new self-relevant information it recognizes in a mirror, typically a colored mark of some kind on its skin that the animal then examines. A related set of tests are those for episodic memory, which are presumed to require projecting oneself into the past in order to re-experience it. Likewise related are tests for future episodic planning, which requires pre-experiencing something by projecting oneself into the future. As with deception and mirror self-identification, the evidence that animals can mentally travel outside of the present remains unclear (Emery & Clayton 2004, Roberts et al. 2008). What all these abilities share in common with the ability to know other minds is the flexible adoption of a point of view that is different (in space, time, or person) from the way one currently experiences the world. As such, they require the ability to make a distinction between world and mind, between objective and subjective. Although several animals can behave in very flexible ways that support some such ability, it remains unclear whether they truly are able to (a) experience a point of view that they deliberately imagine, (b) distinguish this experience from their own experience in the here and now, and (c) derive from this distinction a concept of “mind” of some sort.
Yet in typical adult humans there is no doubt whatsoever that we have knowledge of other minds and our own, and much of the research has focused on the detailed mechanisms that underlie these abilities rather than on demonstrations that we have them at all [although work in infants and children, not treated here, does focus on the age at which these abilities first emerge and how they develop (Blakemore 2008, Striano & Reid 2006)]. Likewise, work in clinical populations such as autism focuses on whether and to what extent they are present (Baron-Cohen 1997, Frith 2001). The mechanisms are of great interest because they seem to require something different from, or additional to, the mechanisms that mediate our knowledge of the shared nonsocial environment. In the case of knowledge of other minds, we appear to begin with much the same information as for nonsocial objects—perception of a face, say—but then go on to make inferences that are unique: We infer emotions, intentions, and beliefs of the other person, none of which we can directly observe because they are internal, relational, or dispositional states in some way. This ability is referred to as “theory of mind” (Leslie 1987, Premack & Woodruff 1978). Our propensity to take this stance toward explaining intentional systems, whether human or not, is influenced by such factors as our motivation to understand a system and to connect with it socially (Epley et al. 2007).
Most puzzling of all is self-knowledge. Unlike the other two forms of knowledge, self-knowledge typically doesn't rely on perceptual observation at all, or at least not on teloreceptive perception. We know what we experience, believe, and think without relying on any observational inference, with the result that we are authoritative about our own minds in a way that other people, whose knowledge of our mind necessarily relies on observational evidence, could never be (which is not to say we are incorrigible on any particular occasion). So what is the source of input that constitutes the evidence on the basis of which we know what is going on in our own minds? One interesting idea is that the source is not sensory at all, but rather is motor in nature. We know what we feel, think, and believe because these are activities that we initiate and about which we can talk to others. This idea has been taken up by some philosophers who emphasize social communication and learning as an essential ingredient to giving content to mental states (Davidson 1987), by neuroscience theories of consciousness that argue sensory consciousness requires relay of information to the prefrontal cortex for action planning (Crick & Koch 1995), and by social neuroscientists who study intentional action and how our sense of agency allows us to understand others as responsible conscious beings (Frith 2007a).
The Consequences for Human Behavior
Although apes have group-specific repositories and transmission of social information that qualify as rudimentary cultures (Whiten et al. 1999), humans alone seem to have language and civilization, and no other mammal has come close to transforming the planet in the way we have. Yet the abilities that underlie this patent social difference remain unclear. Studies showing that great apes are worse than human children on tests of social cognition (Herrmann et al. 2007), especially social learning, even when they are equated with respect to nonsocial cognitive abilities, support the idea that human social cognition is special, perhaps in particular with regard to how we can learn through imitation. However, these studies, like all the others reviewed in the previous section, are heavily debated (for example, it is argued they may be too artificial to demonstrate the social cognitive skills that primates could exhibit in the wild, and solid evidence from field studies is incredibly difficult to obtain).
One set of behaviors that are being intensively investigated by anthropologists, economists, and biologists are those that produce cooperation (Gintis et al. 2003). Chimpanzees appear to have social cognitive abilities that are more adapted to competition than to cooperation (Hare & Tomasello 2004), and they show little spontaneous inclination to help others (Silk et al. 2005). There may be nonreciprocal altruistic behaviors and altruistic punishment (Fehr & Gaechter 2002) that occur only in humans. These abilities depend on a concept of other minds, contribute to reputation and social status, and are critical to aspects of human society and its evolution. We both help and punish others, depending on the circumstances, even when these come at a cost to ourselves and even for nonrelated people, when this is seen as fair, right, or for the greater good. One class of psychological processes that may mediate such behaviors is the moral emotions—strong motivational states, such as pity, pride, or guilt, that link perception of certain classes of social events to actions based on what we judge to be right or wrong.
Social Processes and Social Brain
Controlled and Automatic Processing
The currently dominant view among many cognitive psychologists and neuroscientists proposes two broad sets of processes: those that are controlled and those that are automatic. One could add a third category: those that mediate between controlled and automatic processes. The dichotomous scheme is summarized in a recent review (Lieberman 2007), which enumerates the various properties attributed to controlled and automatic processing. Controlled processes have long been assigned a host of other attributes: They are slow, effortful, reflective, arise late in evolution and development, and often involve language-based declarative reasoning and reflective thinking. Automatic processes are thought to be faster, spontaneous, reflexive, shared in common with a wide range of species and dominant early in development, and often involve emotions. The automatic nature of social cognition has often been stressed, since a large literature supports effects on social judgment and behavior that occur without deliberate reflection (Bargh & Ferguson 2000, Fiske & Taylor 2008). Yet sophisticated views of automaticity acknowledge that, although it is unintentional, automatic processing can be quite diverse and rich in nature (Bargh & Morsella 2008). Regardless of how one carves up the terrain, it seems apparent that both kinds of processes patently contribute to social cognition: Much of it is rapid and fraught with biases and stereotypes of which we may be unaware, consistent with automatic processing; at the same time, a hallmark of human social cognition is our ability to deploy behavior strategically—either to contribute toward the greater good of a society despite selfish inclinations to do otherwise, or to manipulate and deceive others who are trying to predict our behavior.
A large literature has examined the interaction between these two sets of processes. Cognitive control and regulation, abilities that develop relatively late throughout childhood and adolescence, appear to have evolved relatively recently (Braver & Barch 2006). One index of such control is the duration over which a stimulus can be decoupled from an action toward it, such as is seen in temporal discounting of rewards. Such discounting functions are relatively steep for most animals, longer for primates, and longest for humans, who can plan ahead over long time periods to delay obtaining an ultimate reward. Another example of cognitive control is emotion regulation, the ability to alter one's emotional response, expression, and indeed experience, volitionally—a process whose dysfunction in adults contributes to mood disorders (Ochsner & Gross 2005). There is also evidence for interaction in the opposite direction. Theories of decision-making, in particular, have recently argued that automatic, and often emotional, processing influences deliberate choices (Damasio 1994). In a similar vein, studies in the social psychology of stereotyping have shown that our opinions of, and behavior toward, other people is often influenced by covert attitudes that were triggered rapidly and automatically. For instance, social judgments such as trustworthiness can be made from very brief presentations of faces (Bar et al. 2006, Willis & Todorov 2006) that are thought to activate automatic schemas for the rapid, online evaluation of others. One very provocative study found that brief presentations of the faces of real, but unfamiliar, politicians could generate reliable judgments of how competent these politicians looked, without any additional information. Amazingly, such competence judgments based solely on the appearance of a face correlated (weakly but significantly) with real-world election outcomes for those politicians (Todorov et al. 2005).
Dimensions specific to social evaluation have also been proposed: Two universal dimensions of how we perceive and judge other people are competence and warmth (Fiske et al. 2007). These two dimensions capture much about how others might be disposed toward us and thus help us to predict their likely behavior. Perhaps one of the best examples of social cognition that demonstrates the rich interaction between seemingly opposite sets of processes is moral judgment. We judge actions to be right or wrong, and the people who carry them out to be good or bad, based on emotion, inference, automatic and reflective processing, and a host of processes that have evolved to subserve reciprocity, fairness, loyalty, respect, and other behavioral dispositions (Haidt 2007). Many of the distinctions between processes that have been made at the level of cognitive psychology are now being informed by data from neuroscience, which drives home the point of rich interaction even more.
A further consideration regarding the process that subserve social behavior comes from anthropological and comparative data, which can be used to argue for those aspects of social behavior that may be disproportionate to humans, and to provide a corresponding link to those features of the brain that may be disproportionate to humans. We review some of these data in the next section and then turn to the neurobiology.
The Social Brain
The social brain hypothesis attempts to explain the extraordinary size and complexity of the human brain by appeal to particular pressures that a species adapted to social interaction would have had to face, ranging from deception to cooperation to ways of obtaining food and ensuring offspring (Allman 1999; Barrett & Henzi 2005; Dunbar 1998; Dunbar & Schultz 2007a,b). In part, this is a chicken-and-egg question: Did greater general cognitive abilities and intelligence drive our social cognition, or did social cognition enable our intelligence in general (Roth & Dicke 2005)? The evolution of human brain size to its present 1.3 kg is notable for tremendous acceleration on an evolutionarily quite recent timescale, with major increases within less than a million years ago (Ruff et al. 1997). By comparison, the brain size of the great ape species closest in evolution to humans, such as chimpanzees and bonobos, is only 25%–35% of modern human brain size (about the size of the brain our hominid ancestors would likely have had about four million years ago), although body size is comparable. Given the increased maternal investment required to produce offspring with large brains, and the increased metabolic costs of maintaining a large brain (Isler & van Schaik 2006), the central puzzles of human brain evolution are: Why so large, and how could this possibly have taken place so recently?
Responses to these puzzles have often invoked presumptively special aspects of our social behavior. Byrne & Whiten (1988) were among the first to argue in favor of complex social environments as the primary selective pressure for human brain size and later included all aspects of social problem solving, both prosocial and deceitful, in their proposal, the “social brain hypothesis” (Dunbar 1998, Whiten & Byrne 1997). One class of empirical tests for this hypothesis seeks to determine whether those brain regions that differ most in size between humans and apes correspond to regions important for social cognition. Such analyses have pointed to the prefrontal cortex. Though the frontal cortex as a whole is not differentially enlarged in humans as compared to apes (Semendeferi et al. 2002), humans have a comparatively larger frontal polar cortex (Semendeferi et al. 2001) as well as more subtle increases in insular and temporal cortices (Semendeferi & Damasio 2000). Additional empirical tests of the social brain hypothesis focus on operationalizing social complexity in ways that include size of the overall group, size of an average grooming clique, size and frequency of temporally limited subgroups (e.g., coalitions), number and complexity of mating strategies, frequency and complexity of social play, frequency and complexity of deception, and the extent of social learning (Dunbar & Schultz 2007b). Some of these analyses suggest that prevalence of prosocial behaviors, specifically pair bonding behaviors, explain more variance in brain size than do other types of social complexity.
A final point of interest that brings together evolutionary and developmental aspects of human brain size is that humans are highly altricial: The brains of newborns are very immature, and our development, notably including social development, occurs over a protracted period of many years. One way of appreciating this fact is to note that human brains are only about 25% their adult volume at birth—constraints imposed in part by our bipedal nature and the evolution of the female pelvis, the shape of which limits the size of a newborn's head. By comparison, chimpanzee brains are nearly 50% their adult size at birth, and macaque monkey brains are about 70% of their adult size at birth. These differences in the size of the neonatal brain relative to the adult brain mirror the species' differences in the length of their development and their dependency on social support during this development. A recently found skull from a 1.8 million-year-old hominid child provided evidence that our ancestors had a cranial capacity at birth that is essentially like that of apes rather than like that of modern humans. This finding provides further evidence of a change in brain development that occurred relatively recently and that may be one of the features defining the evolution of our species (Coqueugnlot et al. 2004).
Social Modules?
Outlining the brain structures that participate in social cognition raises the question of whether these structures are in any sense specialized for processing social information or whether social cognition is just like cognition in general, only applied to the domain of social behavior. There are some a priori reasons for thinking that we might have evolved specialized systems, because social behavior makes demands that are so unique. It requires rapid identification of social stimuli and signals (such as recognition of people and their dispositions toward us), vast integration of memory (to keep track of who is friend and foe based on past experience), anticipation of others' behavior in a reciprocal and often competitive setting (to generate the unique kind of knowledge outlined in the first section of this review), and the generation of normative evaluations (to motivate social behavior such as altruistic punishment that may be unique to humans and that is required for generating society as we know it). Each of these four examples has been proposed as a unique aspect of human cognition, and one might hypothesize that each is subserved by a specialized evolved ability, or “module” (Barkow et al. 1992, Pinker 1997).
Face Processing and Modularity
One side of an argument about modularity has found responses with a region of the ventral temporal cortex in the fusiform gyrus, dubbed the fusiform face area (FFA), that are larger to faces than to any other visual object category (Kanwisher et al. 1997). The modularity of face processing is further supported by psychological effects unique to faces, such as disruption of processing with inversion, and by single neuron responses in the monkey brain selective to faces (Kanwisher & Yovel 2006, Tsao et al. 2006). However, the FFA also can be activated by nonface objects provided that subjects acquire substantial expertise with them, such as birds, cars, or butterflies in experts for those categories (Gauthier et al. 2000). Although the disproportionate activation by faces argues for a domain-specific module specialized to process a particular category of stimuli (faces) (Kanwisher 2000), the other data argue for a particular type of processing rather than processing for a particular stimulus category (Tarr & Gauthier 2000) (cf. Figure 1). Other imaging data have argued that faces are never represented in a single cortical region, but in a distributed region of cortex considerably more extensive than the FFA (Haxby et al. 2001). However, when competing stimuli are present, as would happen in naturally cluttered environments, the FFA indeed does seem to show a special selectivity for faces (Reddy & Kanwisher 2007).
Modules have been proposed for how we process faces (see sidebar Face Processing and Modularity), for parametrically perceiving genetic relatedness (kinship) (Lieberman et al. 2007), and for detecting people who cheat on social contracts (Cosmides & Tooby 1992), an appealing idea from an evolutionary point of view, since such modules might be expected to facilitate human cooperation, altruistic punishment, and social norm compliance that regulate our ability to function in large groups. A common mechanism thought to mediate between perceptual detection and action is the motivation afforded by strong, often moral, emotions. One example is that the length of cohabitation with a member of the opposite sex calibrates perception of kinship, and correlates with the strength of moral opposition to incest (Lieberman et al. 2003). Moral judgments more generally show many of the features of automatic processing, often appear relatively modular in nature (Hauser 2006), and typically involve strong emotions (Greene & Haidt 2002, Haidt 2001), although it remains unclear whether the emotions are cause or consequence of the judgment. In thinking about the extent to which social cognition might be special in some way, it is useful to distinguish such specialization at the level of the domain of information that is being processed (such as face perception, detailed below) or at the level of the processes that are engaged (whether they are general purpose or special purpose) (Atkinson et al. 2008). This is schematized in Figure 1.
Figure 1.
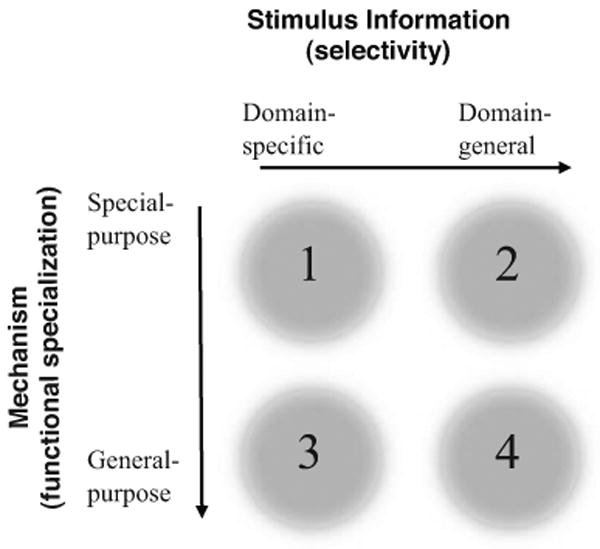
Is social cognition special? Debates about the modularity of social information processing often revolve around the two dimensions shown in this schematic: Is the specialization at the level of processing algorithms (functional specialization) or at the level of the type of information being processed (stimulus selectivity)? A mechanism might be functionally monolithic and apply to a restricted set of stimuli (region 1) or applicable to a large domain of different kinds of stimuli (region 2). Alternatively, a mechanism might contribute to several distinct processes, but in the service of processing either a restricted stimulus class (region 3) or many (region 4). (Modified from Atkinson et al. 2008, Wheeler & Atkinson 2001.)
Neuroscience of Social Cognition
Perceiving Social Stimuli
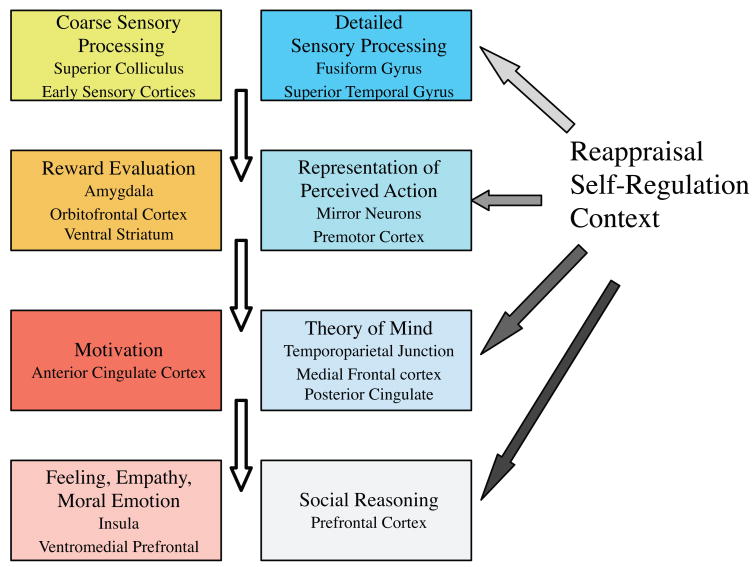
The neural substrates of social cognition (see Figure 2a) are the topic of the rapidly growing field of social cognitive neuroscience (Ochsner 2007, Ochsner & Lieberman 2001), a subdomain of the broader field of social neuroscience (Cacioppo 1994, Cacioppo et al. 2001). One of the earliest reviews to summarize the components of a social brain proposed an initial set of structures thought to be involved in social behavior: the amygdala, the orbitofrontal cortex, and the temporal poles (Brothers 1990). More recent reviews have included additional structures and added putative roles for them (Adolphs 2003, Cacioppo et al. 2007, Fiske & Taylor 2008, Frith 2007b, Frith & Frith 2007, Lieberman 2007). In one scheme (Figure 2b; see color insert), early sensory cortices, as well as subcortical structures such as the amygdala, feed sensory information (in parallel routes) to a mosaic of cortical regions that analyze particular aspects of a stimulus or particular stimulus categories such as faces or bodies.
Figure 2.
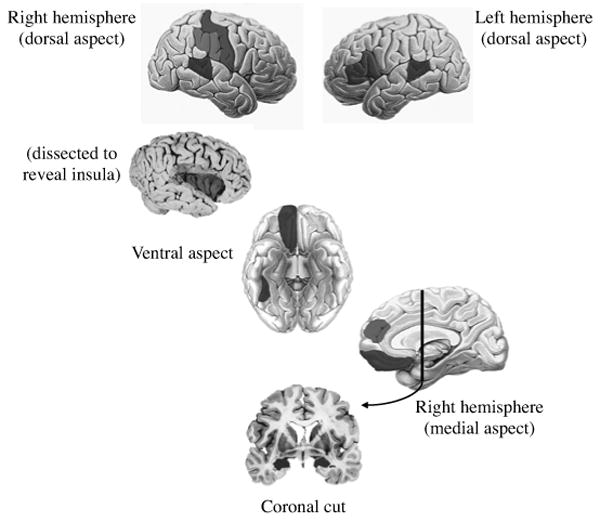
Figure 2a: Processes and brain structures involved in social cognition. Brain structures involved. This is, of course, an incomplete list and emphasizes those structures discussed in the review and outlined in Figure 2b (see color insert). (Top left) A right lateral view of a brain that shows somatosensory cortices and superior temporal gyrus regions; roughly between them and posterior would be the temporoparietal junction, which is not shaded to preserve clarity of the figure. (Top right) Left prefrontal regions are also involved in making personality attributions to others, and indicated again here is the superior temporal gyrus, involved in processes such as biological motion. Below these images are a picture of the insula, revealed when the frontal operculum is removed, and below that, a ventral view of the brain showing medial prefrontal cortex (in this ventral view, medial orbitofrontal cortex) and, more posteriorly, the fusiform gyrus, involved in face processing. Below that, a medial view of the right hemisphere shows the anterior cingulate and again the medial prefrontal cortex. If one takes a coronal section along the line indicated, this cut reveals the amygdala in the medial temporal lobe (very bottom image).
Figure 2b: The schematic outlines a set of processes related more to emotion and empathic simulation (yellow and red boxes, left), and a set of processes related to detailed perception of faces, biological motion, and theory of mind (blue boxes, right). Although there are many examples of processes from the list on the left being distinct from, or in opposition to, processes from the list on the right, the two often complement one another and come into play concurrently. All boxes can be modulated by controlled processing and context, although the extent of this is greatest for the more central processes (different shading of arrows, right). This schematic omits the substantial cross-talk between all of the boxes shown as well as the important role of feedback from “higher” to “lower” structures, part of which is encompassed by the self-regulation and reappraisal modulations (black arrows). (Modified from Adolphs 2003, Adolphs & Spezio 2008.)
At the input end, we know by far the most about how socially relevant information is processed in the visual modality, although progress has been made for audition as well (Belin 2006). There is good evidence for conscious as well as nonconscious routes. The consciously accessible route is thought to depend on visual cortices in the temporal lobe that process object identity and that exhibit some interesting selectivity for social stimuli such as faces (see sidebar Face Processing and Modularity). A subcortical route through the superior colliculus, the mammalian homologue of the optic tectum (the primary visual pathway in amphibians, reptiles, and birds), is thought to be sufficient for visual processing whose results are not consciously accessible. For instance, when face stimuli are shown to one eye while a flickering checker-board pattern is shown to the other eye, viewers are at chance in detecting the face stimulus even though it is present on one retina, and even though different emotional expressions shown on the invisible face stimulus result in differential activation of some brain regions (Jiang & He 2006). Two of these brain regions showing responses to unseen faces are the superior temporal sulcus, a region of visually responsive cortex, and the amygdala, a collection of nuclei in the medial temporal lobe, discussed further below.
Within the modality of touch, there are also distinct processing channels. Some of these, which signal interoceptive bodily information that subserves how we feel, are discussed further below. There also appears to be an exteroceptive channel that, unlike the main touch pathway, does not permit touch discrimination but is able to signal the social-emotional component of touch, such as a caress (Olausson et al. 2002). This pathway appears to rely on particular afferent channels that relay somatosensory information to the insula, a structure involved in affective processing and empathy, which we discuss below. Another sensory modality that may feature distinct channels, but about which relatively little is known in humans, is our sense of smell. In other mammals, there are two primary pathways—one through the olfactory bulb, the other through the vomeronasal system—both of which are involved in social and sexual behavior (Lin et al. 2005). There is some evidence in humans that aspects of our behavior can be influenced by odors without conscious awareness (Stern & McClintock 1998), and activation of brain regions involved in emotion has been found in response to putative pheromone odors (Savic et al. 2001), but the extent to which olfactory social cues play role in everyday life remains unclear.
Evaluating Social Stimuli
The amygdala
The observation (mentioned in the previous section) that stimuli that cannot be consciously perceived still result in discriminative activation of the amygdala, has led to the idea that the amygdala can provide rapid and automatic processing that could bias social cognition. Indeed, its activation is correlated with racial stereotypes of which viewers are unaware (Phelps et al. 2000). Its role in social cognition has been studied most extensively in regard to judgments we make about other people from their faces. Lesion studies found that damage to the amygdala results in an impaired ability to recognize emotional facial expressions (Adolphs et al. 1994), an initial finding that has been followed by a large literature documenting the amygdala's involvement in both appetitive and aversive emotional processing (Aggleton 2000). The amygdala has also been emphasized historically as a structure important for that emotional processing that contributes to social behavior (Kluver & Bucy 1939), another strand in modern research on the amygdala. Recently, at least some of this role has been argued to be due to a more abstract function for the amygdala in general arousal and vigilance (Whalen 1999): It appears to be important to evaluate stimuli as salient because they are unpredictable, because they have been associated with reward or punishment, or because they signal potentially important information. For instance, the impaired recognition of facial expressions of fear in a patient with amygdala lesions (Adolphs et al. 1994) was found to result from an inability to guide one's gaze and visual attention to features in faces normally salient to recognize such expressions, notably the eye region of the face (Adolphs et al. 2005) (Figure 3). Another study found that sequences of unpredictable tones elicited greater amygdala activation, compared with predictable tones, even when no overt rewarding or punishing outcomes were associated with those tones (Herry et al. 2007). These recent findings support earlier ideas that the amygdala is involved in vigilance for stimuli (in all sensory modalities) that are potentially salient because they are ambiguous or unpredictable (Whalen 2007). Other people may exemplify stimuli of that sort.
Figure 3.

Abstract functions of the amygdala contribute to social perception. Bilateral amygdala lesions impair the use of the eyes and gaze to the eyes during emotion judgment. (a) A patient with bilateral damage to the amygdala made significantly less use of information from the eye region of faces when judging emotion. (b) While looking at whole faces, the patient (right column of images) exhibited abnormal face gaze, making far fewer fixations to the eyes than did controls (left column of images). This was observed across emotions (free viewing, emotion judgment, gender discrimination). (c) Magnetic resonance imaging scan of the patient's brain, whose lesion was relatively restricted to the entire amygdala, a very rare lesion in humans. The two round black regions near the top middle of the image are the lesioned amygdalae. (d) When the subject was instructed to look at the eyes (“SM eyes”) in a whole face, she could do this, resulting in a remarkable recovery in ability to recognize the facial expression of fear. The findings show that an apparent role for the amygdala in processing fearful facial expressions is in fact more abstract and involves the detection of, and attentional direction onto, features that are socially informative. (Modified from Adolphs et al. 2005).
The ventromedial prefrontal cortex
Ventral and medial regions of the prefrontal cortex, which encompass a number of interconnected regions that process reward and punishment, regulate emotion, and maintain homeostasis (Öngür & Price 2000), have been linked to social behavior ever since the historical case of Phineas Gage, a nineteenth-century railroad worker who had an iron rod blasted through the front of his head in an accident (Damasio et al. 1994). Not only did Gage survive, but his personality also changed from shrewd, persistent, and respectable to profane, capricious, and unreliable after the accident [although the historical details of this account have been the topic of some debate (MacMillan 2000)]. The association of impairments in social behavior with ventromedial prefrontal cortex (VMPC) damage has since been investigated in much greater detail. Perhaps the most illustrative modern example is patient EVR (Damasio 1994, Eslinger & Damasio 1985). At age 35, EVR underwent resection of a bilateral orbitofrontal meningioma. Most of the VMPC, on both sides of the brain, was lesioned with the tumor resection. Following the surgery, EVR exhibited a remarkable decline in his personal and professional life, including two divorces, the loss of his job, and bankruptcy. Despite the gross alteration of his social conduct and decision-making, neuropsychological testing indicates EVR's intellectual abilities remained unchanged (Saver & Damasio 1991). Subsequent group studies of patients with damage to the VMPC have identified typical personality changes: blunted affect, poor frustration tolerance, impaired goal-directed behavior, inappropriate social conduct, and marked lack of insight into these changes (Barrash et al. 2000). Further experimental work has demonstrated that VMPC damage impairs autonomic responses to emotionally charged pictures (e.g., mutilated bodies, nudes) (Damasio et al. 1990) as well as to emotional memories. Studies involving gambling games indicate that VMPC patients experience diminished emotional arousal before making risky choices (Bechara et al. 1996), as well as diminished regret when considering alternate outcomes after making risky choices (Camille et al. 2004). In such games, patients with lesions to the VMPC persistently make disadvantageous choices. These results support an influential theory about the role of emotion in decision-making (including social decision-making), the so-called somatic marker hypothesis (Damasio 1994, 1996). The hypothesis argues that emotional signals, mediated in part by regions in the VMPC, can be elicited by the anticipation or consideration of the future outcomes of one's actions, and that this signal guides the decision that is made. There has been vigorous debate about whether these emotional signals are conscious or not (Bechara et al. 2005, Maia & McClelland 2004), with the current status being that they need not be conscious in order to influence behavior, although they can be brought into consciousness depending on the task in the experiment (Persaud et al. 2007).
Experimental tests that directly assess social knowledge provide further support for the role of VMPC in social cognition. Patients with VMPC damage have deficits in interpreting nonverbal social information such as facial expression, gestures, or body posture, even though they typically have preserved declarative knowledge of basic social and moral norms. Contextual interpretation of complex social information, such as judging faux pas and sarcasm, as well as aspects of moral judgment, is impaired as well (Beer et al. 2003, Hornak et al. 1996, Koenigs et al. 2007). In particular, damage to the VMPC appears to result in an inability to recognize social faux pas and reduces empathic concern for others (Shamay-Tsoory et al. 2003), an impairment that arises from the emotional contributions made by the VMPC to social cognition as opposed to other factors (such as perspective taking or theory of mind) (Shamay-Tsoory et al. 2005). Studies of moral cognition mentioned elsewhere in this review underscore the importance of VMPC in social decision-making (Koenigs et al. 2007, Moll et al. 2005).
Although the majority of studies have focused on, and the largest effects have been found for, patients who have bilateral damage to the VMPC, unilateral damage also causes the pattern of impairments described above, only milder. There appears to be an interesting asymmetry in that unilateral right-sided lesions seem to cause a more severe impairment than do unilateral left-sided lesions, an effect that was also seen in one of the studies cited above (Shamay-Tsoory et al. 2005). A further wrinkle on this story is that unilateral right lesions are more severe than left in males, whereas unilateral left lesions may be more severe than right in females (Tranel et al. 2005).
Patients with early-onset damage involving VMPC are a unique resource for investigating the development of social cognition. Like patients with adult-onset damage, individuals acquiring VMPC damage in infancy or early childhood manifest defects in social conduct and decision-making despite intact language, memory, and IQ. However, the social defects following early-onset VMPC damage appear more severe than in the adult-onset cases. Common features include apathy and unconcern; lack of guilt, empathy, or remorse; violent outbursts; lewd and irresponsible behavior; and petty criminal behavior together with a profound lack of awareness of these behavioral problems (Anderson et al. 2000). Unlike adult-onset cases, early-onset VMPC patients may have impaired knowledge of social and moral conventions (Anderson et al. 1999, 2000). These results indicate that the VMPC is critically involved in the acquisition of social and moral knowledge during development. Adult-onset VMPC patients, who presumably undergo normal social development, retain declarative access to social facts, but they appear to lose access to emotional signals that are necessary to guide appropriate on-line social and decision-making behavior in real-life situations. Early-onset VMPC patients seem to have never acquired appropriate levels of factual social knowledge in the first place, nor do they have access to normal online emotional processing, resulting in an even greater level of social impairment.
Empathy and simulation
One feature of human cognition is a rerepresentation of both sensory and motor information in order to permit more flexible behavior. For instance, a remapping of interoceptive information about the state of one's own body may allow humans and other primates to construct explicit representations of how they feel, and to know and consequently regulate how they feel in a flexible way. This remapping has been proposed to rely on relays of interoceptive processing into the insula, and a further remapping within the anterior insula is thought to consolidate body-state information about oneself with social and contextual information to provide a neural substrate of the conscious experience of emotions (Craig 2002, 2008). This region of the brain has been found to be activated in a large number of studies that involve other people, or information about other people, as the stimuli. For instance, observing the hand of a loved receive a painful electric shock will activate the insula in the brain of the perceiver (Singer et al. 2004). This and other studies have tied the insula not only to the experience of one's own emotions, but also to the empathic feeling of others' emotions: one way in which we know what is going on inside other people is to simulate aspects of what is happening in their brain (Keysers & Gazzola 2007). Associating our observations of other people with representations of our own internal states, motivations, and intentions is hypothesized to be a general mechanism whereby we are able to generate knowledge of other minds (Keysers & Perrett 2004).
Mirroring other people can be entirely automatic, go unnoticed, and form one basis for learning about the world through others. For instance, the amygdala we discussed above has been classically shown to be necessary for acquiring Pavlovian fear conditioning, but it also turns out to be important for learning to fear a stimulus merely by observing another person experience its consequences (Olsson et al. 2007)—an effect that, like classical fear conditioning, can take place even when the stimuli cannot be consciously perceived (Olsson & Phelps 2004). In a study with rats, a naive observer rat that had not been subjected to aversive stimuli of any kind nonetheless showed discriminatory activation within the amygdala when it interacted with another rat, depending on whether or not that other rat had experienced electric shock (Knapska et al. 2006). These findings are in line with a large literature in social psychology confirming that we automatically and often nonconsciously pick up social signals from others. When we become aware that these signals are signals, more uniquely human forms of social cooperativity and deception may appear, and the knowingly shared conscious experience opens up forms of social learning on which culture can build (Frith & Frith 2007).
Empathy and emotion do not only include feelings, but they also motivate us to act, for instance when empathy causes sympathy (de Vignemont & Singer 2006). In its most schematic form, information would be expected to flow from high-level sensory representations that contribute to conscious experience of the world and our bodies, to high-level premotor representations that motivate action. The anterior cingulate cortex is one structure that is thought to receive high-level information about expected and actual sensory events, to monitor conflicts (Botvinick et al. 2004), and to integrate this with emotional information to motivate behavior (Craig 2008). It is activated in a number of experiments in which strong emotional information [such as pain (Vogt 2005) or social exclusion (Eisenberger et al. 2003)] lead to an interruption of ongoing processing and motivate a behavioral change (Devinsky et al. 1995). It appears to play a role at a high level of behavioral regulation in that it can adjust general learning about environmental contingencies when their reliability changes through time (Behrens et al. 2007)—presumably also an important role in updating our social information from other people.
Several other regions within the prefrontal cortex are routinely activated when people experience strong emotions and when they are motivated to take actions based on those emotions. These regions are all connected with the anterior cingulate cortex, include dorsolateral as well as ventromedial sectors of prefrontal cortex, and have been implicated in reward-based learning and instrumental behavior in both cooperative and competitive social interactions. They have also been highlighted as implementing one way in which emotions can motivate moral, altruistic, and socially regulatory behaviors (Damasio 1994, 2003). For instance, a network of orbitofrontal and dorsolateral prefrontal cortex is activated when punishment by others induces social norm compliance (Spitzer et al. 2007), and lesions to the ventromedial prefrontal cortex result in impaired social emotions, impaired social functioning in the real world, and an abnormal skew toward making utilitarian moral judgments when moral emotions and rational considerations are in conflict (Koenigs et al. 2007).
Emotions motivate behavior; thus, simulating other people's emotions provides us with one strategy for predicting what they are likely to do. A complementary strategy is to simulate aspects of the premotor representations that would normally accompany goal-directed behavior, a mechanism supported by finding representations, at the systems and cellular level (Gallese et al. 2004, Rizzolatti & Craighero 2004), that are engaged both when we plan to execute an action ourselves and when we observe another person carry out the same action. Although some of these “mirror” representations respond only to viewing a very specific action, the majority can abstract from the particulars of any specific action or even sensory modality to encode goal-directed intentions (Fogassi et al. 2005). Together, our ability to simulate motivational and premotor representations of other people may ground our ability to know about other minds (Gallese 2007), although deliberative reasoning (as formulated in classical theory-of-mind accounts) no doubt also plays a role. The extent to which these two processes, automatic simulation and more deliberately reflecting on mental states, come into play appears to depend on the demands of a task—their engagement is thus to some extent context-dependent (de Lange et al. 2008). It is also interesting to note that monkeys have such so-called “mirror neurons” but do not imitate or appear to know about other minds, indicating that additional enabling mechanisms, possibly including enculturation, are required for mere mirroring at the neural level to generate knowledge of other minds (Iriki 2006). Although historically it has been seen as distinct from simulation, theory-of-mind ability, broadly construed, encompasses several distinct strategies and several neural regions with a single goal: to understand the internal states that predict the behavior of other people. In fact, one may consider the outputs of a simulation/mirroring system as the potential inputs to a mentalizing/theory-of-mind system: We may first generate motor representations of how another person is performing an action (via simulation and mirroring) and then use this representation in more flexible ways to infer the reasons and intentions behind the observed action (Keysers & Gazzola 2007).
Here we find another argument regarding modularity: the idea that our ability to reason about the minds of others, theory of mind, is an encapsulated, modular process of some kind (Leslie 1987). Theory-of-mind tasks, which ask subjects to reason about the intentions and beliefs of others, activate medial prefrontal cortex and the temporoparietal junction (TPJ). Complex biological motion that signals animacy activates high-level visual regions at the interface between processing streams for object identification (which includes the FFA; see sidebar Face Processing and Modularity) and visually guided action in the posterior superior temporal cortex (Schultz et al. 2005). This region is adjacent to, and one of the likely sources of input to, the TPJ, which in turn is involved in taking different spatial perspectives as well as the perspective of another person when we have to imagine their beliefs. The argument about the modularity of the TPJ arises from findings, on the one hand, that lesions within it impair the ability to attribute beliefs to others (Samson et al. 2004) and that it is activated selectively when we imagine the beliefs of somebody else (Saxe 2005), versus findings, on the other hand, that it is also activated when we redirect our attention in nonsocial tasks (Mitchell 2007).
There is less debate about the role of the medial prefrontal cortex in theory-of-mind abilities, as it is consistently activated when we think about other people's internal states (Amodio & Frith 2006, Saxe & Powell 2006). This region is activated when we need to infer the current beliefs of another person, evaluate their longer-term traits and dispositions, and when we think about our own minds. In fact, it is also activated when we think about the minds of animals (Mitchell et al. 2005). In short, it appears to come into play whenever we think about the mind at all, something that we may do spontaneously when we are not engaged with the external world (Buckner & Carroll 2006, Mitchell et al. 2002). Another region activated in theory-of-mind tasks and likely involved in generating knowledge of both our own mind and the minds of others is the posterior cingulate cortex (Saxe & Powell 2006), a region that shows functional coupling with the medial prefrontal cortex at rest.
Modulating Social Cognition: Context and Regulation
It is likely that a similar story obtains for stimuli in all sensory modalities: There is processing that contributes to what we are conscious of, as well as processing that operates below the level of conscious reportability and discrimination; different properties of stimuli are processed in partly segregated but parallel processing streams; and this sensory processing is then associated with a variety of factors that determine its saliency and ultimately influence its deployment toward behavior. This largely feed-forward view of processing needs to be tempered by the fact that there is massive feedback everywhere in the brain, structurally often greater than the feed-forward projections. For instance, the amygdala projects back to all levels of cortical visual processing, those from which it receives input as well as earlier ones from which it does not, positioning it to influence visual information processing in a global fashion (Freese & Amaral 2005). Some of this feedback from “higher” to “lower” structures also implements aspects of controlled processing, such as emotion regulation (indicated by separate arrows in Figure 2b, although it in fact arises from some of the structures shown, notably the prefrontal cortex).
Social behavior depends critically on context and intention, a sensitivity that arises from the rich interplay between controlled and automatic processing of social information, and a modulation long emphasized within social psychology (Todorov et al. 2006). One way of viewing such modulations is to think of an initial feed-forward sweep of social information processing that is rapid and automatic, followed by cycles of additional processing that are biased by the first, but modulated by top-down effects that may incorporate controlled processing and conscious intent (Cunningham & Zelazo 2007). There are numerous examples at all levels of processing showing how contextual information modulates, or even gates, social information processing. At the sensory perceptual level, information about faces is processed differently depending on context. Thus, a surprised face can be interpreted as looking afraid or looking happy, depending on a preceding sentence (Kim et al. 2004). Afraid and angry faces are interpreted differently depending on whether their gaze is direct or averted (Adams & Kleck 2003). Some context modulates what we counterfactually expect might happen. Thus, in the example of social norm compliance, brain structures associated with strong emotions are activated only when the subject knows that punishment is possible, not when it is known to be impossible (Spitzer et al. 2007). An important and common finding (often utilized as a control condition in imaging studies) is that knowing that a particular event or outcome was intentionally caused by another person leads to a different interpretation than knowing that the event was unintentional or was caused by a computer. Thus, in the case of the negative emotions and anterior cingulate activation induced by social exclusion, this obtains only when the subject is convinced that other people are volitionally excluding him or her, not when the “exclusion” is explained as a technical malfunction of some sort (Eisenberger et al. 2003). What we know about people from their past behavior provides an important context that modulates our responses to, and actions toward, others. In studies of empathy, it was found that our perception of other people's fairness (from their behavior in an economic game) modulated how much empathy was felt when they were observed to be given painful electric shock, an effect that correlated with activation of the insula (Singer et al. 2006).
Emotional responses can be modulated not only by context, but also volitionally by reinterpreting a situation, or indeed solely by willful control. This is effortful, develops relatively late in childhood and adolescence, and depends on the prefrontal cortex (Ochsner & Gross 2005). Although it is somewhat simplistic, one useful heuristic is that more anterior regions within prefrontal cortex can exert cognitive control over successively posterior regions (Koechlin et al. 2003), an idea consistent with the role of frontal polar cortex (Brodmann's area 10, the most-anterior part of the brain) in overriding ongoing processing to explore new options in nonstationary environments (Daw et al. 2006). Interestingly, as we reviewed above, frontal polar cortex also appears to be a region that has expanded the most in human evolution (Semendeferi et al. 2001), and it is a region activated when we need to explicitly represent another person's mind as distinct from our own or the state of the world (Amodio & Frith 2006). Such a role may be critical to social communication, cooperation, and deception, and it may be unique to humans (Saxe 2006).
Another distinction that can be made is between sustained and volitional control on the one hand, and interruption of ongoing processing triggered by monitoring conflict on the other. These two functions have been argued to be subserved by dorsolateral regions of the prefrontal cortex and the anterior cingulate cortex, respectively (Miller & Cohen 2001). Cognitive control can extend to explicit regulation of one's own thoughts: One entertaining study found evidence for these two structures in sustained and transient suppression of forbidden thoughts (about a white bear in the experiment) (Mitchell et al. 2007). Other examples of the role of the dorsolateral prefrontal cortex in cognitive control abound. For instance, it is activated when shorter-term reward (which activates reward-related regions such as the ventral striatum and medial frontal cortex) must be foregone in lieu of longer-term reward (McClure et al. 2004). It is also activated in moral judgment tasks when an emotionally prepotent moral judgment must be overridden (in the fashion that Kant had in mind) to arrive at the decision that is best in terms of aggregate welfare (Greene et al. 2004). Moral dilemmas that pit strongly emotional outcomes against equally strong utilitarian considerations (e.g., smothering one's baby to prevent it from crying and giving away a group of people hiding in wartime) engage substantial cognitive conflict, and people do not give unanimous answers to such dilemmas. The proportion of cold utilitarian answers (e.g., smothering the baby) is increased by damage to regions that normally engage strong social emotions, such as the ventromedial prefrontal cortex (Koenigs et al. 2007), a finding we noted above. One could speculate that damage to the dorsolateral prefrontal cortex might result in the converse impairment: a larger proportion of emotional deontological answers (e.g., not smothering the baby, because this is felt to be too abhorrent and one cannot override the strong emotional aversion). The way in which our laws assign blame and dole out punishment also captures an important context effect: an interaction between the harmful consequences of an action, and the belief and intention of the person carrying it out. When examining good or bad consequences (e.g., somebody drank poison and died or drank water and lived) interacting with belief (e.g., the person offering the drink believed it was poison or did not), the results showed a strong interaction of the outcome with the belief. This interaction corresponded to activation of the TPJ (Young et al. 2007), a region discussed above in the representation of another mind's belief.
Interpretation of context and degree of control vary from person to person, and so it is perhaps not surprising that substantial individual differences exist in many of the processes and structures discussed above. In the case of empathy and the insula, individual differences exist on empathy questionnaires that correlate with the degree of insula activation. In the case of the amygdala, individual differences in anxiety correlate with amygdala activation to facial expressions, and there are now some intensively investigated genetic polymorphisms that are know to influence amygdala activation and may predispose to psychiatric illness (Meyer-Lindenberg & Weinberger 2006, Skuse 2006). One particularly interesting story is a polymorphism in a gene that affects the level of the neurotransmitter serotonin in the brain (known to be involved in affiliative behaviors and influenced by drugs such as Prozac and ecstasy). The polymorphism (corresponding to two different but relatively common alleles) correlates with mood disorders and modulates the strength of cognitive control over amygdala processing by the anterior cingulate cortex, likely a substrate of emotion regulation (Pezawas et al. 2005).
Conclusion
Although many open questions remain, several of them linked to technical issues in measurement and analysis (see sidebar Future Challenges), it seems clear that human social cognition is both special and ubiquitous. It draws on many of the same brain structures involved in perception, cognition, and behavior more generally, but specialization may be evident at the level of neural processing as well (see sidebar Face Processing and Modularity). What then is it that distinguishes human social cognition from that of other species? Three prominent differences discussed above are: the ability to shift one's conscious experience to places and times outside the here-and-now, and into the viewpoint of another mind (Buckner & Carroll 2006, Suddendorf & Corballis 1997); the association of our evaluation of others with strong moral emotions that motivate particular aspects of social behavior, such as altruistic punishment (Fehr & Gaechter 2002); and the ability to use these abilities flexibly as a function of context, across considerable time intervals, and with the help of a prodigious episodic memory that helps us to keep track of a large number of other individuals and their past behavior (Stevens et al. 2005). When the demands on social cognition become severe, these three abilities taken together may define much of the nature of human conscious experience and indeed provide an argument for its emergence.
Future Challenges
To understand the function of a neural structure, we need to know all its inputs and outputs, a description that is difficult to obtain in humans but becoming possible in some animal models. For instance, how olfactory information about a mate interacts with reward systems during mating to result in pair-bonding behavior of prairie voles has been worked out in spectacular detail (Insel & Young 2001, Young & Wang 2004). Two recent technical developments in magnetic resonance imaging are beginning to sketch such a picture also in humans: Diffusion imaging is providing information about the structural connectivity of the human brain, and functional connectivity modeling is providing estimates of information flow between structures; a currently hot area of development is integrating these two sources of connectivity information (Friston et al. 2003, Jbabdi et al. 2007). One functional network is the so-called default or resting-state network, first identified on the basis of positron emission tomography studies and thought to be active during rest, deactivated when we process external stimuli or engage in an externally directed task (Gusnard & Raichle 2001), and subserving processes that include perspective taking and self-reflection (Buckner & Carroll 2006). It may be one aspect of the automatic human propensity to think about what might happen, or what will happen in the future, in order to prepare ourselves and plan our behavior (Bar 2007). It is also intriguing to note that people with autism, who are impaired in social functioning, do not activate this same network at rest (Kennedy et al. 2006).
Summary Points
Inferring what is going on inside other people's minds from their observed behavior may be a uniquely human ability, although other primates show precursors to this ability.
The ability to infer others' mental states is thought to be an important contributor to human culture and civilization.
Although many different psychological processes contribute to social cognition, they are often grouped into two broad categories: those related to automatic processing driven more by the stimuli and those related to controlled processing driven more by the person's goals and intentions.
Social information processing looks in many respects different from nonsocial information processing. This has provided support for some schemes that claim social information processing is modular.
The amygdala is a structure in the medial temporal lobe important to regulating social behavior and recognizing emotional facial expressions. However, recent work suggests its role is quite abstract and not specific to social cognition.
The orbitofrontal cortex is a region of cortex in the frontal lobes that is involved in reward processing. Lesions of this region in humans result in severe impairments in real-life social behavior despite cognition in other domains that is otherwise relatively intact.
The insula is a region of cortex buried underneath the frontal cortex that is involved in representing states of our own body, such as pain. It is also involved when we feel empathy for others, such as when we observe somebody else in pain.
Social cognition is sensitive to context, and the brain regions involved in social cognition are modulated in their activation by social context and volitional regulation.
Two hypotheses about how we infer other people's mental states are that we do so by simulation and empathy (abilities that involve regions such as the premotor cortex and the insula) or via more deliberate theory-of-mind abilities (which involve regions such as the medial prefrontal cortex and the temporoparietal junction).
Acknowledgments
This review was supported by grants from the National Institute of Mental Health, the Simons Foundation, and the Gordon and Betty Moore Foundation. I thank Phillipe Schyns, Joanne Silk, and Susan Fiske for helpful comments on the manuscript.
Footnotes
Disclosure Statement: The author is not aware of any biases that might be perceived as affecting the objectivity of this review.
Literature Cited
- Adams RB, Kleck RE. Perceived gaze direction and the processing of facial displays of emotion. Psychol Sci. 2003;14:644–47. doi: 10.1046/j.0956-7976.2003.psci_1479.x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behavior. Nat Rev Neurosci. 2003;4:165–78. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R. How do we know the minds of others? Domain-specificity, simulation, and enactive social cognition. Brain Res. 2006;1079:25–35. doi: 10.1016/j.brainres.2005.12.127. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]; This study found that a patient with bilateral amygdala lesions was impaired in recognizing fear in facial expressions because patient failed to fixate the eyes in faces and thus failed to use facial information normally needed to recognize fear.
- Adolphs R, Spezio M. The neuroscience of social cognition. In: Cacioppo JT, Berntson G, editors. Handbook of Neuroscience for the Behavioral Sciences. New York: Wiley; 2008. In press. [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Aggleton J, editor. The Amygdala. A Functional Analysis. New York: Oxford Univ. Press; 2000. [Google Scholar]
- Allman JM. Evolving Brains. New York: Sci. Am. Library; 1999. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–37. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Tranel D, Damasio AR. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol. 2000;18:281–96. doi: 10.1207/S1532694202Anderson. [DOI] [PubMed] [Google Scholar]
- Atkinson AP, Heberlein AS, Adolphs R. Are people special? A brain's eye view. In: Adams RB, Nakayama K, Shimojo S, editors. The Science of Social Vision. New York: Oxford Univ. Press; 2008. In press. [Google Scholar]
- Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11:280–89. doi: 10.1016/j.tics.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6:269–78. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Ferguson MJ. Beyond behaviorism: on the automaticity of higher mental processes. Psychol Bull. 2000;126:925–45. doi: 10.1037/0033-2909.126.6.925. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Morsella E. The unconscious mind. Perspect Psychol Sci. 2008;3:73–79. doi: 10.1111/j.1745-6916.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkow JH, Cosmides L. In: The Adapted Mind: Evolutionary Psychology and the Generation of Culture. Tooby J, editor. New York: Oxford Univ. Press; 1992. [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press; 1997. p. 200. [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–81. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi P. The social nature of primate cognition. Proc Biol Sci. 2005;272:1865–75. doi: 10.1098/rspb.2005.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio A. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Neurosci. 2005;9(4):159–62. doi: 10.1016/j.tics.2005.02.002. [DOI] [PubMed] [Google Scholar]; Part of a debate about whether emotional biases in decision making are conscious.
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. Regulatory functions of self-conscious emotion: insights from patients with orbitofrontal damage. J Personal Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nat Neurosci. 2007;10:1214–21. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Belin P. Voice processing in human and nonhuman primates. Philos Trans R Soc Lond B Biol Sci. 2006;361:2091–107. doi: 10.1098/rstb.2006.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Boesch C. What makes us human (Homo sapiens)? The challenge of cross-species comparison. J Comp Psychol. 2007;121:227–40. doi: 10.1037/0735-7036.121.3.227. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. Extracting core components of cognitive control. Trends Cogn Sci. 2006;10:529–32. doi: 10.1016/j.tics.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11(2):49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]; Reviews common brain networks engaged when we daydream, recollect the past, imagine the future, and imagine other people's minds.
- Byrne R, Whiten A, editors. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford: Clarendon; 1988. [Google Scholar]
- Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31:113–28. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Amaral DG, Blanchard JJ, Cameron JL, Carter CS, et al. Social neuroscience: progress and implications for mental health. Perspect Psychol Sci. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Adolphs R, Carter CS, Davidson RJ, et al., editors. Foundations in Social Neuroscience. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–70. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. How Monkeys See the World. Chicago, IL: Univ. Chicago Press; 1990. [Google Scholar]
- Coqueugnlot H, Hublin JJ, Vellon F, Houet F, Jacob T. Early brain growth in Homo erectus and implications for cognitive ability. Nature. 2004;431:299–332. doi: 10.1038/nature02852. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J. Cognitive adaptations for social exchange. In: Barkow JH, Cosmides L, Tooby J, editors. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. New York: Oxford Univ. Press; 1992. pp. 163–228. [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Feldman-Barrett L, editors. Handbook of Emotions. 3rd New York: Guilford; 2008. pp. 272–88. [Google Scholar]
- Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–23. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Looking for Spinoza: Joy, Sorrow, and the Feeling Brain. Orlando, FL: Harcourt; 2003. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–4. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davidson D. Proceedings and Addresses of the American Philosophical Association. Vol. 61. New York: Oxford Univ. Press; 1987. Knowing one's own mind; pp. 441–58. [Google Scholar]
- Daw N, O'Doherty JP, Dayan P, Seymour B, Dolan RJ. Polar exploration: cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–79. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–57. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:436–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- de Waal F, Dindo M, Freeman CA, Hall MJ. The monkey in the mirror: hardly a stranger. Proc Natl Acad Sci USA. 2005;102:11140–47. doi: 10.1073/pnas.0503935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dunbar RI. The social brain hypothesis. Evol Anthropol. 1998;6:178–90. [Google Scholar]
- Dunbar RI, Schultz S. Evolution in the social brain. Science. 2007a;317:1344–47. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Dunbar RI, Schultz S. Understanding primate brain evolution. Philos Trans R Soc Lond B Biol Sci. 2007b;362:649–58. doi: 10.1098/rstb.2006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–92. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Clayton NS. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science. 2004;306:1903–7. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- Epley N, Waytz A, Cacioppo JT. On seeing human: a three-factor theory of anthropomorphism. Psychol Rev. 2007;114:864–86. doi: 10.1037/0033-295X.114.4.864. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fehr E, Gaechter S. Altruistic punishment in humans. Nature. 2002;415:137–40. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]; We punish others whose behavior we deem to be unfair, even when such punishment is at a cost to ourselves.
- Fiske ST, Cuddy AJC, Glick P. Universal dimensions of social cognition: warmth and competence. Trends Cogn Sci. 2007;11:78–83. doi: 10.1016/j.tics.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Fiske ST, Taylor SE. Social Cognition: From Brains to Culture. 3rd New York: McGraw-Hill; 2008. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–67. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J Comp Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny WD. Dynamic causal modeling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Frith CD. Making Up the Mind: How the Brain Creates Our Mental World. New York: Blackwell Sci; 2007a. [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc Lond B Biol Sci. 2007b;362:671–78. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–32. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–79. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Gallese V. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond B Biol Sci. 2007;362:659–69. doi: 10.1098/rstb.2006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Chimpanzees: self-recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–97. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gintis H, Bowles S, Boyd R, Fehr E. Explaining altruistic behavior in humans. Evol Hum Behav. 2003;24:153–72. [Google Scholar]
- Greene JD, Haidt J. How (and where) does moral judgment work? Trends Cogn Sci. 2002;6:517–23. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle MA. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haidt J. The emotional dog and its rational tail: a social intuitionist approach to moral judgment. Psychol Rev. 2001;108:814–34. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- Haidt J. The new synthesis in moral psychology. Science. 2007;316:998–1002. doi: 10.1126/science.1137651. [DOI] [PubMed] [Google Scholar]
- Hare B, Tomasello M. Chimpanzees are more skillful in competitive than in cooperative cognitive tasks. Anim Behav. 2004;68:571–81. [Google Scholar]
- Hauser MD. Moral Minds: How Nature Designed Our Universal Sense of Right and Wrong. New York: HarperCollins; 2006. [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representation of faces and objects in ventral temporal cortex. Science. 2001;293:2425–29. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Herrmann E, Call J, Hernandez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science. 2007;317:1360–66. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, DiSalle F, Perrig WJ, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; The amygdala responds to temporally unpredictable (jittered) tones, even when these are not emotional.
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioral changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–35. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]; A review of the best-understood model system for bonding and affiliative behaviors: the sexual behavior of voles.
- Iriki A. The neural origins and implications of imitation, mirror neurons and tool use. Curr Opin Neurobiol. 2006;16:660–67. doi: 10.1016/j.conb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Isler K, van Schaik CP. Metabolic costs of brain size evolution. Biol Lett. 2006;2:557–60. doi: 10.1098/rsbl.2006.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Woolrich MW, Andersson JL, Behrens TE. A Bayesian framework for global tractography. Neuroimage. 2007;37:116–29. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He S. Cortical responses to invisible faces: dissociating subsystems for facial-information processing. Curr Biol. 2006;16:2023–29. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Domain specificity in face perception. Nat Neurosci. 2000;3:759–63. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–28. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–96. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Perrett DI. Demystifying social cognition: a Hebbian perspective. Trends Cogn Sci. 2004;8:501–7. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–97. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Knapska E, Nikolaev E, Boguszewski P, Walasek G, Blaszczyk J, et al. Between-subject transfer of emotional information evokes specific pattern of amygdala activation. Proc Natl Acad Sci USA. 2006;103:3858–62. doi: 10.1073/pnas.0511302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–85. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, et al. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–11. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie A. Pretense and representation: the origins of “theory of mind”. Psychol Rev. 1987;94:412–26. [Google Scholar]
- Lieberman D, Tooby J, Cosmides L. Does morality have a biological basis? An empirical test of the factors governing moral sentiments relating to incest. Proc R Soc Lond B Biol Sci. 2003;270:819–26. doi: 10.1098/rspb.2002.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Tooby J, Cosmides L. The architecture of human kin detection. Nature. 2007;445:727–31. doi: 10.1038/nature05510. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposes that detecting genetic relatedness constitutes a module.
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–77. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- MacMillan M. An Odd Kind of Fame: Stories of Phineas Gage. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Maia TV, McClelland JE. A re-examination of the evidence for the somatic marker hypothesis: what participants really know in the Iowa gambling task. Proc Natl Acad Sci USA. 2004;101:16075–80. doi: 10.1073/pnas.0406666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Miklosi A, Kubinyi E, Topal J, Gacsi M, Viranyi Z, Csanyi V. A simple reason for a big difference: Wolves do not look back at humans, but dogs do. Curr Biol. 2003;13:763–66. doi: 10.1016/s0960-9822(03)00263-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2007;18(2):262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage. 2005;28:757–62. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Macrae CN. Separating sustained from transient aspects of cognitive control during thought suppression. Psychol Sci. 2007;18:292–97. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Ochsner KN. Social cognitive neuroscience: historical development, core principles, and future promise. In: Kruglanski A, Higgins ET, editors. Social Psychology: A Handbook of Basic Principles. 2nd New York: Guilford; 2007. pp. 39–66. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotions. Trends Cogn Sci. 2005;9:242–49. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. Am Psychol. 2001;56:717–34. [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]; A patient lost the ability to discriminate touch but could feel a caress as pleasant, a finding that suggests a “social touch” channel.
- Olsson A, Nearing KI, Phelps EA. Learning fears by observing others: the neural systems of social fear transmission. Soc Cogn Affect Neurosci. 2007;2:3–11. doi: 10.1093/scan/nsm005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol Sci. 2004;15:822–28. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]; Shows that humans can learn to fear stimuli through direct experience (classical Pavlovian conditioning), through observation (social learning), and through instruction (unique to humans). However, only the first two can take place nonconsciously.
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. On the lack of evidence that nonhuman animals possess anything remotely resembling a “theory of mind”. Philos Trans R Soc Lond B Biol Sci. 2007;362:731–44. doi: 10.1098/rstb.2006.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud N, LcLeod P, Cowey A. Post-decision wagering objectively measures awareness. Nat Neurosci. 2007;10:257–61. doi: 10.1038/nn1840. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O'Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, et al. Performance on indirect measures of race evaluation predicts amygdala activation. J Cogn Neurosci. 2000;12:729–38. doi: 10.1162/089892900562552. [DOI] [PubMed] [Google Scholar]
- Pinker S. How the Mind Works. New York: Norton; 1997. [DOI] [PubMed] [Google Scholar]
- Plotnik JM, de Waal F, Reiss D. Self-recognition in an Asian elephant. Proc Natl Acad Sci USA. 2006;103:17053–57. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povinelli DJ, Vonk J. Chimpanzee minds: suspiciously human? Trends Cogn Sci. 2003;7:157–60. doi: 10.1016/s1364-6613(03)00053-6. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–26. [Google Scholar]
- Reddy L, Kanwisher N. Category selectivity in the ventral visual pathway confers robustness to clutter and diverted attention. Curr Biol. 2007;17:2067–72. doi: 10.1016/j.cub.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc Natl Acad Sci USA. 2001;98:5937–42. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, MacPherson K, Petter M, McMillan N, Musolino E. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320:113–15. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–57. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Trinkaus E, Holliday TW. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387:173–76. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly I, Humphreys G. Left temporoparietal junction is necessary for representing someone else's belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saver JL, Damasio AR. Preserved access and processing of social knowledge in a patient with acquired sociopathy due to ventromedial frontal damage. Neuropsychologia. 1991;29:1241–49. doi: 10.1016/0028-3932(91)90037-9. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activation in humans. Neuron. 2001;31:661–68. doi: 10.1016/s0896-6273(01)00390-7. [DOI] [PubMed] [Google Scholar]
- Saxe R. Hybrid vigour: reply to Mitchell. Trends Cogn Sci. 2005;9:364. [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–39. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–99. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schultz J, Friston KJ, O'Doherty JP, Wolpert DM, Frith C. Activation in posterior superior temporal sulcus parallels parameters inducing the percept of animacy. Neuron. 2005;45:625–35. doi: 10.1016/j.neuron.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–41. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J Hum Evol. 2000;38:317–32. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Lu A, Schenker N, Damasio H. Humans and great apes share a large frontal cortex. Nat Neurosci. 2002;5:272–77. doi: 10.1038/nn814. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Silk JB. Social components of fitness in primate groups. Science. 2005;317:1347–51. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli D, et al. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437:1357–59. doi: 10.1038/nature04243. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–69. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D. Genetic influences on the neural basis of social cognition. Philos Trans R Soc Lond B Biol Sci. 2006;361:2129–41. doi: 10.1098/rstb.2006.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer M, Fischbacher U, Hermberger B, Groen G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56:185–96. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Stern K, McClintock MK. Regulation of ovulation by human pheromones. Nature. 1998;392:177–79. doi: 10.1038/32408. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Cushman F, Hauser M. Evolving the psychological mechanisms for cooperation. Annu Rev Ecol Evol Syst. 2005;36:499–518. [Google Scholar]
- Striano T, Reid VM. Social cognition in the first year. Trends Cogn Sci. 2006;10:471–76. doi: 10.1016/j.tics.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis M. Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr. 1997;123:133–67. [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–69. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Todorov A, Harris LT, Fiske ST. Toward socially inspired social neuroscience. Brain Res. 2006;1079:76–85. doi: 10.1016/j.brainres.2005.12.114. [DOI] [PubMed] [Google Scholar]
- Todorov A, Mandisodza AN, Goren A, Hall CC. Inferences of competence from faces predict election outcomes. Science. 2005;308:1623–26. doi: 10.1126/science.1110589. [DOI] [PubMed] [Google Scholar]
- Tomasello M. The Cultural Origins of Human Cognition. Cambridge, MA: Harvard Univ. Press; 1999. [Google Scholar]
- Tomasello M, Call J, Hare B. Chimpanzees understand psychological states—the question is which ones and to what extent. Trends Cogn Sci. 2003;7:153–56. doi: 10.1016/s1364-6613(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg N, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–81. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–74. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1999;7:177–87. [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends Cogn Sci. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Wheeler M, Atkinson A. Domains, brains and evolution. In: Walsh DM, editor. Naturalism, Evolution and Mind. Cambridge, UK: Cambridge Univ. Press; 2001. pp. 239–66. [Google Scholar]
- Whiten A, Byrne R, editors. Machiavellian Intelligence II: Extensions and Evaluations. Cambridge, UK: Cambridge Univ. Press; 1997. [Google Scholar]
- Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, et al. Cultures in chimpanzees. Nature. 1999;399:682–85. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychol Sci. 2006;17:592–98. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Wood JN, Glynn DD, Phillips BC, Hauser M. The perception of rational, goal-directed action in nonhuman primates. Science. 2007;317:1402–5. doi: 10.1126/science.1144663. [DOI] [PubMed] [Google Scholar]
- Young LJ, Cushman F, Hauser MD, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci USA. 2007;104:8235–40. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]