Abstract
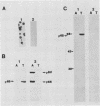
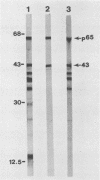
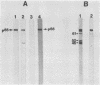
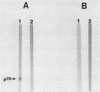
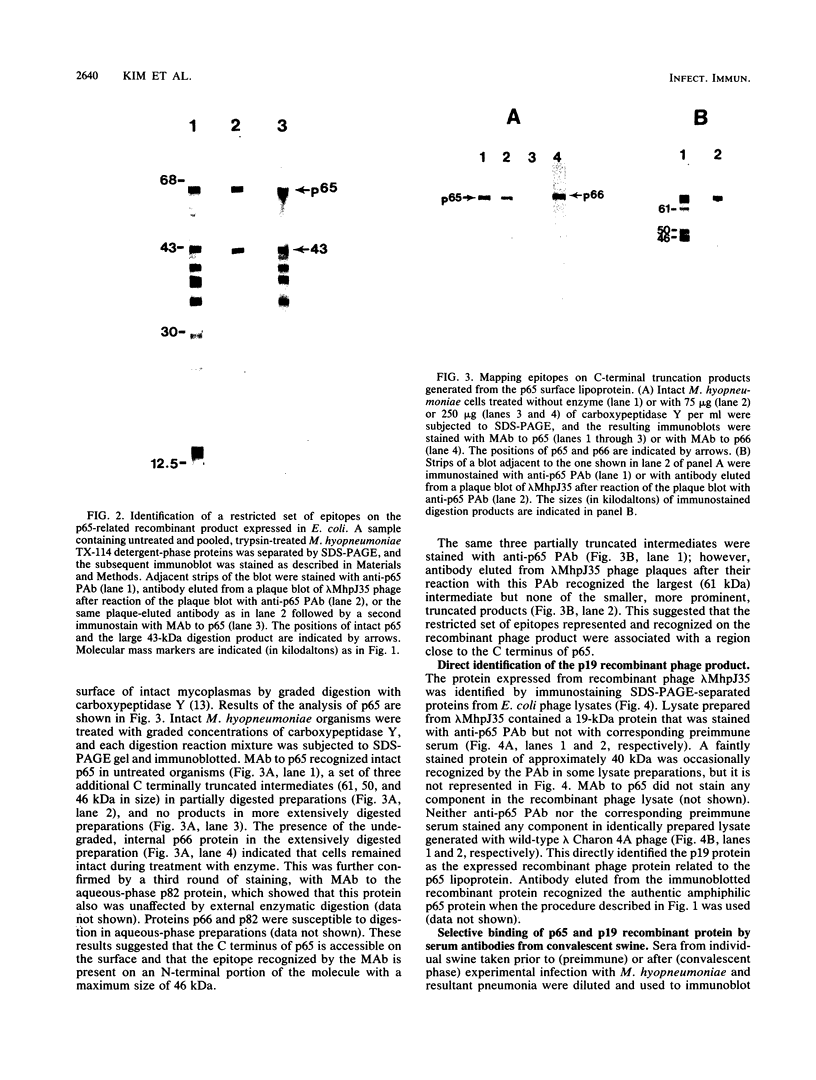
A previously characterized lipid-modified amphiphilic surface protein of Mycoplasma hyopneumoniae, p65, has been defined by its reaction with a surface-binding monoclonal antibody (MAb) and by its exclusive partitioning into the detergent phase during Triton X-114 phase fractionation (K. S. Wise and M. F. Kim, J. Bacteriol. 169:5546-5555, 1987). In the current study, polyclonal mouse antibody (PAb) to gel-purified p65 was used to identify recombinant phage plaques expressing p65-related epitopes. Several characteristic partial tryptic fragments of p65 were recognized by both PAb and p65 and MAb to p65, but the PAb population specifically eluted from recombinant phage plaques bound only epitopes restricted to the largest of these fragments. Graded carboxypeptidase-Y digestion of intact M. hyopneumoniae generated C terminally truncated peptides that were recognized by PAb to p65 and MAb to p65, indicating that the C terminus and much of the adjoining region of p65 were present and accessible on the external face of the membrane. However, antibody eluted from recombinant phage plaques bound only to the largest truncated polypeptide, suggesting that a recombinant product corresponding to the C-terminal region of p65 was expressed in Escherichia coli. A 19-kilodalton recombinant protein (p19), which was recognized by PAb to p65 but not by MAb to p65, was detected in recombinant phage lysates. Serum antibodies from swine taken after, but not before, experimentally induced M. hyopneumoniae pneumonia preferentially recognized the native, amphiphilic p65 lipoprotein and also bound specifically to the p19 recombinant product. This confirmed that the p65 lipoprotein is a major immunogen of M. hyopneumoniae recognized during disease and identified its C-terminal region as an immunogenic domain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker T. M., Boyer M. J., Keith J., Watson-McKown R., Wise K. S. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect Immun. 1988 Feb;56(2):295–301. doi: 10.1128/iai.56.2.295-301.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., DeOgny L., Slaughter C., Radolf J. D., Norgard M. V. Acylation of the 47-kilodalton major membrane immunogen of Treponema pallidum determines its hydrophobicity. Infect Immun. 1989 Sep;57(9):2878–2885. doi: 10.1128/iai.57.9.2878-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl C. E., Dahl J. S., Bloch K. Proteolipid formation in Mycoplasma capricolum. Influence of cholesterol on unsaturated fatty acid acylation of membrane proteins. J Biol Chem. 1983 Oct 10;258(19):11814–11818. [PubMed] [Google Scholar]
- Freeman M. J., Armstrong C. H., Sands-Freeman L. L., Lopez-Osuna M. Serological cross-reactivity of porcine reference antisera to Mycoplasma hyopneumoniae, M. flocculare, M. hyorhinis and M. hyosynoviae indicated by the enzyme-linked immunosorbent assay, complement fixation and indirect hemagglutination tests. Can J Comp Med. 1984 Apr;48(2):202–207. [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hsu P. L., Chamberlain N. R., Orth K., Moomaw C. R., Zhang L. Q., Slaughter C. A., Radolf J. D., Sell S., Norgard M. V. Sequence analysis of the 47-kilodalton major integral membrane immunogen of Treponema pallidum. Infect Immun. 1989 Jan;57(1):196–203. doi: 10.1128/iai.57.1.196-203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert M. Q., Herrmann R., Schaller H. Surface proteins of Mycoplasma hyopneumoniae identified from an Escherichia coli expression plasmid library. Infect Immun. 1985 Aug;49(2):329–335. doi: 10.1128/iai.49.2.329-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarnicola S. M., McIntosh M. A., Wise K. S. Multiple translational products from a Mycoplasma hyorhinis gene expressed in Escherichia coli. J Bacteriol. 1990 Jun;172(6):2986–2995. doi: 10.1128/jb.172.6.2986-2995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström S., Johansson K. E., Wieslander A. Selective acylation of membrane proteins in Acholeplasma laidlawii. Eur J Biochem. 1986 Apr 1;156(1):85–94. doi: 10.1111/j.1432-1033.1986.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Wise K. S. Phenotypic switching in mycoplasmas: phase variation of diverse surface lipoproteins. Science. 1990 Jan 19;247(4940):315–318. doi: 10.1126/science.1688663. [DOI] [PubMed] [Google Scholar]
- Ross R. F., Zimmermann-Erickson B. J., Young T. F. Characteristics of protective activity of Mycoplasma hyopneumoniae vaccine. Am J Vet Res. 1984 Oct;45(10):1899–1905. [PubMed] [Google Scholar]
- Schouls L. M., Mout R., Dekker J., van Embden J. D. Characterization of lipid-modified immunogenic proteins of Treponema pallidum expressed in Escherichia coli. Microb Pathog. 1989 Sep;7(3):175–188. doi: 10.1016/0882-4010(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Swancutt M. A., Radolf J. D., Norgard M. V. The 34-kilodalton membrane immunogen of Treponema pallidum is a lipoprotein. Infect Immun. 1990 Feb;58(2):384–392. doi: 10.1128/iai.58.2.384-392.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., McIntosh M. A., Robbins J., Wise K. S. Cloned genomic DNA sequences from Mycoplasma hyorhinis encoding antigens expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4154–4158. doi: 10.1073/pnas.80.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Kim M. F. Major membrane surface proteins of Mycoplasma hyopneumoniae selectively modified by covalently bound lipid. J Bacteriol. 1987 Dec;169(12):5546–5555. doi: 10.1128/jb.169.12.5546-5555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Watson R. K. Mycoplasma hyorhinis GDL surface protein antigen p120 defined by monoclonal antibody. Infect Immun. 1983 Sep;41(3):1332–1339. doi: 10.1128/iai.41.3.1332-1339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wróblewski H., Nyström S., Blanchard A., Wieslander A. Topology and acylation of spiralin. J Bacteriol. 1989 Sep;171(9):5039–5047. doi: 10.1128/jb.171.9.5039-5047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T. F., Ross R. F. Assessment of antibody response of swine infected with Mycoplasma hyopneumoniae by immunoblotting. Am J Vet Res. 1987 Apr;48(4):651–656. [PubMed] [Google Scholar]