Abstract
Purpose
By controlling membrane potential and intracellular free Ca2+ ([Ca2+]i), K+ currents are pivotal in the regulation arterial smooth muscle tone. The goal of the present study was to identify and characterise the A-type K+ current in retinal microvascular smooth muscle (MVSM) and to examine its role in modulating membrane potential and cellular contractility.
Methods
Whole-cell perforated patch-clamp recordings were made from MVSM cells within intact isolated arteriolar segments. Prior to patch-clamping, retinal arterioles were anchored down in the physiological recording bath and perfused with an enzyme cocktail to remove surface basal lamina and to electrically uncouple endothelial cells from overlying MVSM cells.
Results
K+ currents were activated by depolarising steps from -80 mV to +100 mV in 20 mV increments. A dominant, non-inactivating Ca2+-activated K+ current was elicited by depolarisation to potentials positive of -50 mV. Inhibition of this current by 100 nM Penitrem A revealed a rapidly inactivating K+ current which resembled an A-type current. The A-type current was insensitive to tetraethylammonium (TEA) at 1 mM, but was partially suppressed by higher concentrations (10 mM). 4-aminopyridine (10 mM; 4-AP) completely blocked the A-type current. The 4-AP sensitive transient current activated at a potential of -60 mV with peak current densities averaging 29.7 ± 5.68 pA/pF at +60 mV. The voltage of half-inactivation was -28.3 ± 1.9 mV and the time constant for recovery from inactivation at +60 mV was 118.7 ± 7.9 ms. Under current-clamp conditions 4-AP depolarised the membrane potential by ∼3-4 mV and triggered small contractions and relaxations of individual MVSM cells within the walls of the arterioles.
Conclusions
A-type current is the major voltage-dependent K+ current in retinal MVSM and appears to play a physiological role in suppressing cell excitability and contractility.
Introduction
The contractile activity, or vascular tone, of microvascular smooth muscle (MVSM) cells in the walls of retinal arterioles is the major determinant of resistance to blood flow through the retinal circulation1. Retinal arterioles, therefore, have a primary physiological role in controlling blood pressure and local tissue perfusion in the retina. Since the retinal vasculature lacks any obvious autonomic nerve supply2, retinal MVSM tone is largely dependent on the complex interplay of vasodilator and vasoconstrictor stimuli released from neighbouring endothelial and retinal cells3. These signals evoke changes in MVSM contractility by influencing the level of free intracellular [Ca2+]i available to the contractile apparatus4,5. Retinal MVSM cells have multiple mechanisms for regulating their intracellular Ca2+ concentrations including influx6, efflux7 as well as Ca2+ release and uptake by the sarcoplasmic reticulum (SR)5. Plasmalemmal ion channels play an important role in the regulation of [Ca2+]i both by providing pathways for Ca2+ entry and by regulating MVSM cell membrane potential. Such channels are key agents, therefore, in the control of [Ca2+]i and contractility.
K+ currents are important physiological regulators of membrane potential in arterial smooth muscle cells8. The opening of K+ channels in the cell membrane increases K+ efflux, leading to membrane hyperpolarisation. This closes voltage-dependent Ca2+ channels, which decreases Ca2+ influx and results in vasodilatation9. There are four main classes of K+ channels in the arterial smooth muscle cell membrane, namely ATP-sensitive K+ channels (KATP), calcium-activated K+ channels (KCa), voltage-activated K+ channels (KV) and inward rectifier K+ channels (KIR). All of these are believed to participate in the regulation of MVSM tone10.
KV channels are the most numerous and diversified ion channel structures11. However, KV currents may be broadly categorised as either slow “delayed rectifier” currents or rapid “A-type” currents according to their time dependent features. Delayed rectifier currents exhibit a delayed onset of activation followed by little or slow inactivation, while A-type currents are typically distinguished by their rapid rates of inactivation12. A-type currents are classically associated with neurons where they are thought to play a role in regulating firing frequency13,14. A-type currents have also been identified in macro- and microvascular smooth muscle cells15-18, but their physiological role has yet to be fully elucidated12.
In the present study we have, for the first time, identified a rapidly inactivating K+ current in retinal MVSM which has several electrophysiological and pharmacological properties consistent with an A-type current, but also demonstrates some novel features. Current-clamp studies were also performed and these suggest that this current plays a physiological role in retinal MVSM, increasing the membrane potential and thereby reducing excitability.
Methods
Retinal arteriole preparation
Male Sprague-Dawley rats (200-300 g) were anaesthetized with CO2 and killed by cervical dislocation. Animal use conformed to the guidelines of the ARVO statement for the Use of Animals in Ophthalmic and Vision Research and UK Home Office Regulations. Retinae were rapidly removed and arterioles, devoid of surrounding neuropile, isolated as previously described1,5,6. In brief, retinae were lightly triturated using a fire polished Pasteur pipette (internal tip diameter 0.3 mm) in a low Ca2+ Hanks’ solution. Homogenates were centrifuged at 2800 rpm (952g) for 1 min, the supernatant aspirated off and the tissue washed again with low Ca2+ medium. The suspension was then stored at 21°C until needed. Arteriolar segments remained useable for up to 10 h under these conditions.
Electrophysiology
Homogenate was placed in a 2 ml recording bath on the stage of an inverted microscope for 5 min. Arteriolar segments were visually distinguished from venules by the presence of a thick wall of circularly arranged smooth muscle cells and lack of abluminal pericytes. Arterioles were anchored down with tungsten wire slips (50 μm diameter, 2 mm length) and superfused with normal Hanks’ solution at 37°C. Enzyme and drug solutions were delivered via a 7-way micro-manifold with an exchange time of ∼ 1 s, as measured by switching to a dye solution. Prior to electrophysiological recording, vessels were digested for 20-min with an enzyme cocktail of collagenase 1A (0.1 mg/ml) and protease type XIV (0.01 mg/ml) to remove surface basal lamina and to electrically uncouple endothelial cells from overlying arteriolar smooth muscle cells (Fig 1). Cells treated in this manner remained viable as confirmed by their ability to exclude trypan blue solution (0.04% in normal Hanks’ solution) for periods >2 hours (n=6). As a further measure of cell viability, retinal MVSM cell [Ca2+]i was measured by fura-2 microfluorimetry as previously described5,6. No changes in [Ca2+]i were observed following 20-min enzyme digestion (basal [Ca2+]i was 72.4 ± 11.5 nM before and 65.4 ± 9.9 nM afterwards; n=5; p=0.44, paired t-test).
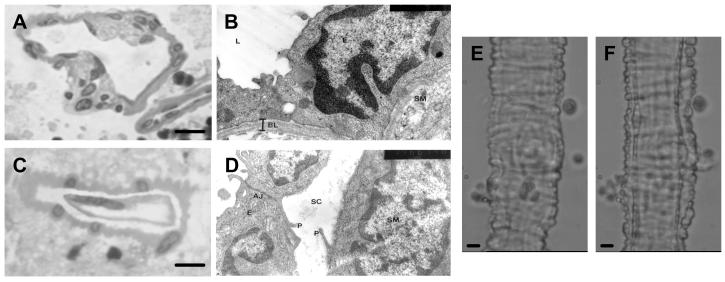
Figure 1.
A. Light micrograph of an untreated retinal arteriole stained with toluidine blue (converted to greyscale). The endothelial cells and smooth muscle cells are in tight apposition throughout the cross-section. Scale bar = 5 μm B. Electron micrograph at ×40,000 magnification of an untreated retinal arteriole. The basal lamina (BL) can be clearly seen between the endothelial cells (E) and the smooth muscle (SM). C. Light micrograph of a retinal arteriole treated with collagenase and protease for 20 min. The endothelial cells have completely pulled away from the overlying smooth muscle layer creating a 1-5 μm separation cleft. Scale bar = 5 μm D. Electron micrograph at ×28,000 magnification of a retinal arteriole treated with enzyme for 20 min. Remnants of digested basal lamina can be seen in the separation cleft (SC). Projections (P) normally connecting the endothelial cells to the smooth muscle layer have broken away. AJ, adheren junction. E. Photomicrograph of an untreated retinal arteriole anchored down in the physiological recording bath. Scale bar = 5 μm. F. The same vessel following 20 min digestion with collagenase and protease. The separation of the endothelial cells from the smooth muscle layer can be clearly visualised. Scale bar = 5 μm.
Ionic currents were recorded from retinal MVSM cells whilst still embedded within their parent arterioles. Arterioles used for the experiments were 25-40μm in diameter, and in order to guarantee adequate space clamping of the electrically coupled MVSM cell layer, recordings were restricted to microvessels ≤500 μm in length19,20. Current and voltage-clamp experiments were performed using the whole-cell perforated patch clamp technique21 with an Axopatch-1D patch clamp amplifier (Axon Instruments, USA). Electrodes (1-2 MΩ in free bathing solution) were pulled from filamented borosilicate glass capillaries (1.5 mm o.d. w 1.17 mm i.d., Clark Electromedical Instruments, UK). Internal pipette solutions were K+ based with amphotericin B as the perforating agent (see solutions below). Recordings were delayed until full perforation of the membrane patch had been achieved, as judged from the development of repeatable currents in response to step depolarizations: this usually took 3-5 min. Liquid junction potentials (< 2 mV) were compensated electronically. Series resistance (34.5 ± 3.86 MΩ; n=15) and cell capacitance (14.2 ± 0.5 pF; n=15) were usually uncompensated. Recordings were low pass filtered at 0.5 kHz and sampled at 2 kHz by a National Instruments PC1200 interface using software provided by J. Dempster (University of Strathclyde, UK). Leakage currents were subtracted off-line from the active currents with the use of the standard leak subtraction protocol contained within the Patch software suite. For determination of whole-cell current densities, cell membrane capacitance was determined from the time constant of a capacitance transient elicited by a 20 mV depolarization from -80 mV with a sampling frequency of 20 kHz.
For current-clamp experiments, digital video recordings of the arterioles were collected simultaneously. This was accomplished by attaching a video camera (WAT-902B, Watec, Japan) to the side-port of the inverted microscope and the signal captured online via an A/D converter (Data Translation, US) and stored on a computer.
Solutions and drugs
The bath solution had the following composition (in mM): 140, NaCl; 6, KCl; 5, D-glucose; 2, CaCl2; 1.3, MgCl2; 10, HEPES, pH 7.4 with NaOH. Low Ca2+ medium differed only in that it contained 0.1 mM CaCl2. For perforated patch-clamp recordings the pipette contained (mM): 138 KCl; 1 MgCl2; 0.5 EGTA; 0.2 CaCl2; 10 Hepes (pH adjusted to 7.2 using NaOH; free Ca2+: 100 nM) to which 600 μg ml-1 amphotericin B was added.
Amphotericin B, collagenase 1A, penitrem A, protease type XIV, tetraethylammonium chloride (TEA), 4-aminopyridine (4-AP) and 9-anthracene carboxylic acid (9-AC) were purchased from Sigma (Poole, UK). The effects of channel inhibitors were deemed to be through a direct action on the MVSM since we have previously shown that drug agents gain limited access to the endothelial cells when applied to the outside of the vessels5. In addition, delamination of the vessels in the present study further restricts the likelihood of drugs mediating their effects through endothelial cell release of putative diffusible messengers.
Data Handling
Data are reported as the mean ± S.E.M, and n denotes the number of arterioles from which recordings were made. Curve fitting was performed in an iterative fashion using Sigmaplot V8 (Systat Software, UK). S.E.Ms for biophysical data were calculated from curve fits for individual arterioles.
Results
Pharmacological isolation of the A-type current
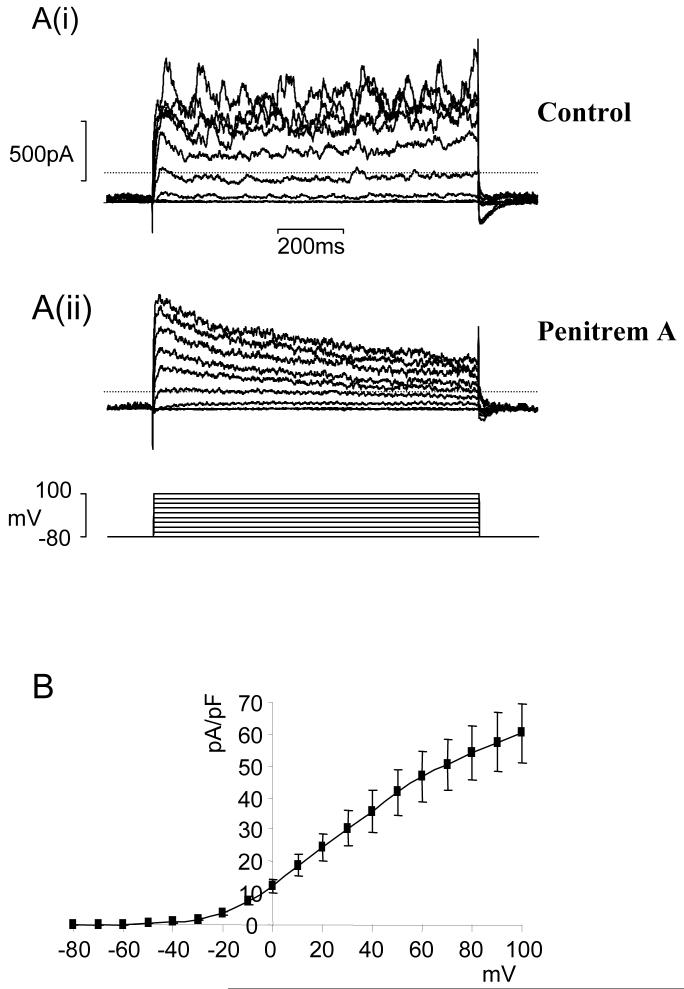
Outward currents were measured in MVSM cells still embedded in retinal arteriolar fragments using the perforated patch-clamp technique. Patch pipettes were K+-filled and 9-AC (1 mM) was included in the external bathing solution to minimize contamination from Ca2+-activated Cl- currents. Application of depolarising voltage steps with 20 mV increments from a holding potential of -80 mV elicited an outward current that was composed of an initial transient and then sustained component (Fig 2A(i)). Spontaneous transient outward currents (STOCs) were superimposed on the background of the current and these became more pronounced at increasingly positive membrane potentials. The complex kinetics of the net outward current suggested that it may be formed from multiple components. Addition of 100 nM Penitrem A, a potent inhibitor of large conductance Ca2+-activated K+ channels (BKCa)22, reduced the sustained current and abolished STOCs revealing a rapidly inactivating current which resembled an A-type current (Fig 2A(ii)). This transient current persisted during exposure to Ca2+-free/EGTA solution (n=4), suggesting that it was Ca2+-independent and was unaffected by the KATP channel inhibitor, glibenclamide (1 μM; n=6). In all subsequent voltage-clamp experiments, 100 nM Penitrem A was added to the external bathing solution to eliminate BKCa current.
Figure 2.
A family of whole-cell currents in a representative retinal arteriole. Currents were elicited by 1-sec depolarising steps from a holding potential of -80 mV in the absence (Ai) and presence (Aii) of 100 nM Penitrem A. B. Average peak current density as a function of voltage for the Penitrem A insensitive current (n=10).
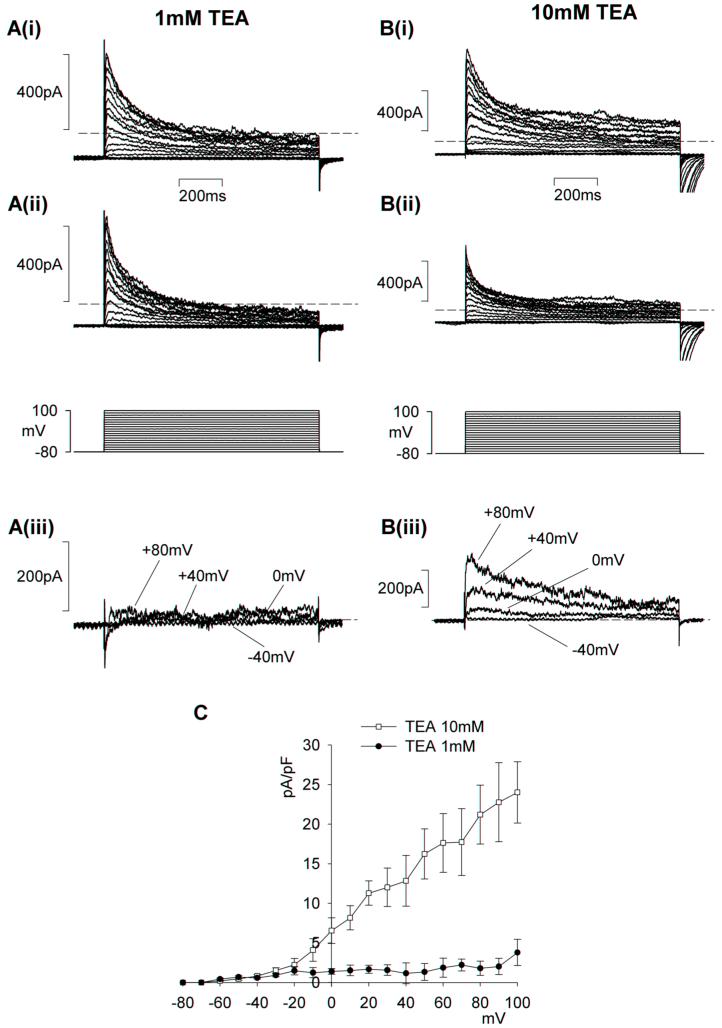
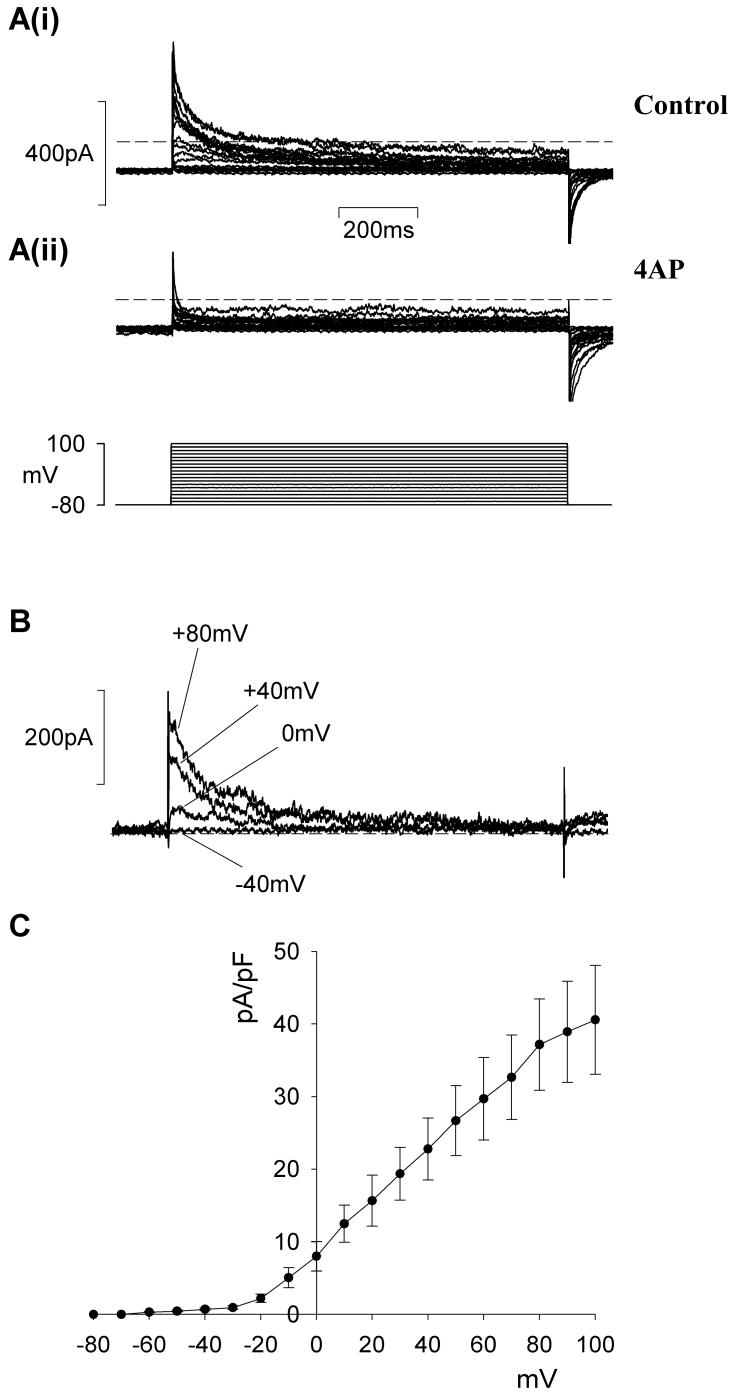
In other types of smooth muscle the A-type current often coexists with delayed rectifier current12. These currents can be separated from one another on the basis of their respective sensitivities to 4-AP and TEA. In retinal MVSM cells, low levels of TEA (1 mM) had no effect on the A-type current, but partially inhibited the residual sustained current (Fig 3A,C). External TEA at higher concentrations (10 mM) caused some depression of the peak A-type current (Fig 3B,C). It was evident from these data that the A-type and delayed rectifier currents in retinal MVSM could not be fully separated on the basis of their differential sensitivity to TEA. In contrast, we found that the currents could be isolated by applying 4-AP (10 mM). 4-AP caused nearly complete inhibition of peak A-type current, whereas the sustained, delayed rectifier current was unaffected (Fig 4A). The 4-AP-difference currents were characterised by a rapid rise and peak which then subsided back to baseline levels (Fig 4B). An average peak current-voltage relationship for the 4-AP-sensitive A-type current recorded in 9 arterioles is plotted in Fig 4C. Peak current density at +60 mV was 29.7 ± 5.68 pA pF-1.
Figure 3.
Effects of tetraethylammonium (TEA) on retinal MVSM transient outward currents. The membrane potential was stepped for 1-sec from -80 to +100 mV in 10 mV increments. A & B, whole cell A-type currents before (i) and after (ii) 1 and 10 mM TEA, respectively. iii. Difference currents for the individual TEA concentrations obtained by subtracting ii from i at the voltage steps indicated. Dashed lines mark zero current. C. average peak current-density relationships for the difference currents in 1 mM (n=6) and 10 mM (n=5) TEA.
Figure 4.
Effect of 4-AP on retinal arteriole A-type currents. A. Whole-cell currents before (i) and after (ii) external 4-AP (10 mM). B. Difference currents obtained by subtracting i from ii at the voltage steps specified. Dashed lines denote zero current. C. average peak current density as a function of voltage for the 4-AP sensitive current (n=9).
Biophysical properties
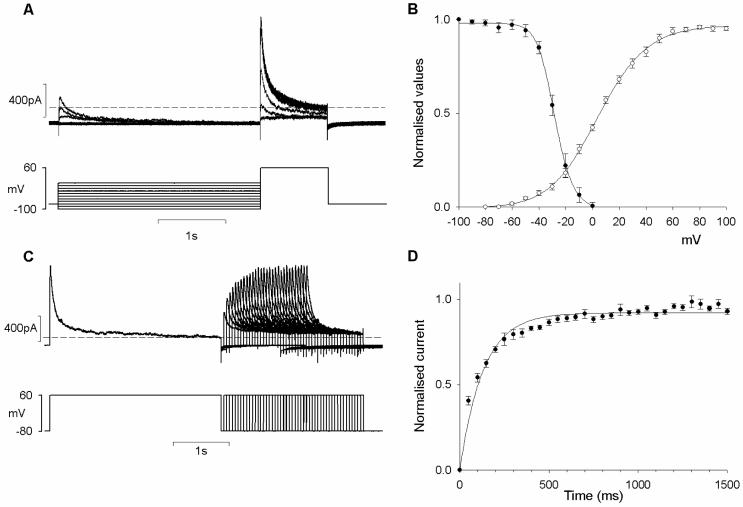
Our pharmacological data with 4-AP indicated that the A-type current in retinal MVSM is closely approximated by the peak minus the sustained components of the net outward current in Penitrem A. Using this method, we investigated the biophysical properties of the A-type current in retinal MVSM cells. The voltage dependence of inactivation was investigated by holding the retinal arterioles at different membrane potentials during a conditioning pre-pulse and then applying a common test pulse (+60 mV). The amplitude of the peak current during the test pulse decreased as the conditioning potential was increased from -100 mV to 0 mV (Fig 5A). The peak current flowing during each test pulse was expressed relative to the maximum current recorded after the conditioning pre-pulse at -100 mV (I/I max) and the data was fitted with a Boltzmann function. The voltage for half-inactivation of the A-type current was -28.3 ± 1.9 mV. The fact that the channels underlying the A-type current in retinal MVSM only began to inactivate at the threshold potential for activation of the delayed rectifier current (-50 mV, see Fig 3C, 1 mM TEA) precluded a full separation of these components based on their voltage sensitivities. This differs from the situation in other types of smooth muscle, where the A-type and delayed rectifier currents can be separated by shifting the pre-pulse holding potential from -80 to -40 mV23.
Figure 5.
Electrophysiological properties of the A-type current. A. A double pulse protocol was applied to obtain the steady state voltage dependent inactivation. Membrane currents were measured at +60 mV following 3-sec conditioning potentials ranging from -100 to 0 mV. B. Voltage dependence of activation and inactivation measured in 10 arterioles. Normalised conductance (G/Gmax) is plotted against membrane potential. C. Recovery from inactivation of the A-type current using the voltage protocol depicted at the bottom. D. Average time course of the recovery from inactivation in 10 vessels. Dashed lines mark zero current in A & C.
To examine the voltage dependency of activation (Fig 5B) peak currents were converted to a conductance using the following equation: G=I/(Vm-Ek), where I was the current amplitude, Vm was the command potential and Ek was the equilibrium potential for potassium (Ek= -80 mV). Values were then normalised to the maximum conductance (G/Gmax). Fitting this data with a Boltzmann function gave a voltage of half-activation of -6.1 ± 1.6 mV. The activation and inactivation curves overlap substantially between -60 and 0 mV, revealing a relatively large ‘window’ of steady-state A-type current within this voltage range (Fig 5B).
Recovery from inactivation was studied using a double-pulse protocol with conditioning and test pulses to +60 mV from the holding potential of -80 mV (Fig 5C). The interval between the end of the conditioning pulse and the test pulse was increased and the peak A-type current during test depolarisations was then normalised to that during the conditioning pulse. The summary data has been plotted as a function of the recovery interval (Fig 5D). The time course of recovery from inactivation was well fitted with a single exponential with a time constant of 118.7 ± 7.9 ms.
Physiological function
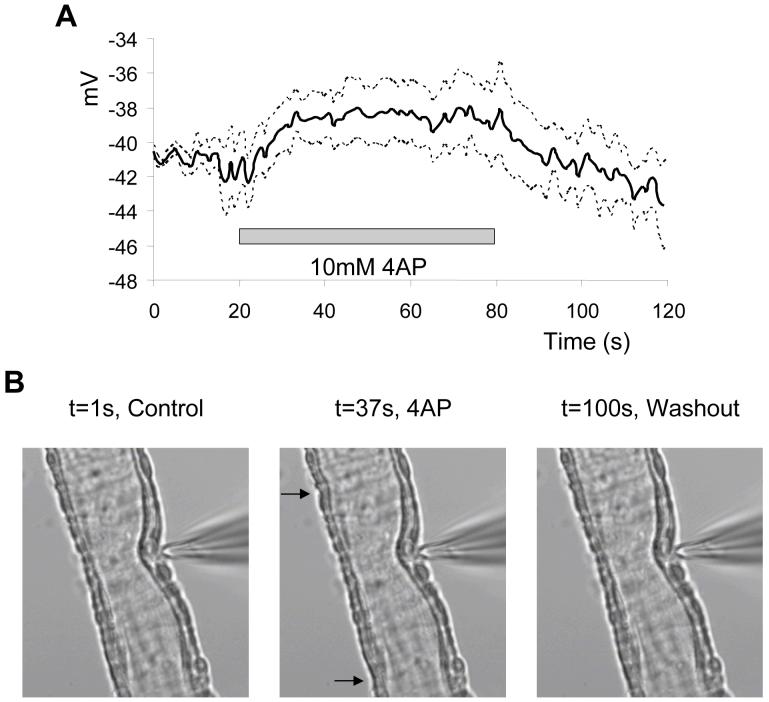
In the final series of experiments we investigated the contribution of the A-type current to retinal MVSM cell membrane potential and contractility. In current-clamp mode and in the absence of Penitrem A and 9-AC, the membrane potential of retinal arterioles was set to -40 mV, close to the reported resting membrane potential for ocular and distal cerebral MVSM cells measured using intracellular recording electrodes24,25. Without injection of negative current, resting membrane potentials were low (∼-15 mV), presumably reflecting the effects of MVSM-endothelial cell uncoupling. 4-AP (10 mM) depolarized retinal arterioles by ∼3-4 mV (Fig 6A). Despite the small level of depolarization, simultaneous video recordings revealed that 4-AP triggered miniature contractions and relaxations of individual MVSM cells within walls of the arterioles (Fig 6B), suggesting that electrical and mechanical excitability was increased when the A-type current was inhibited. No effects on membrane potential or MVSM cell contractility were observed when current-clamped vessels (at -40 mV) were exposed to 100 nM Penitrem A alone (n=6).
Figure 6.
Effects of 4-AP on electrical and mechanical activity. A. Retinal MVSM cell membrane potential measured in current-clamp mode before, during and after washout of 4-AP. Solid black line represents the average trace obtained from 5 arterioles and the dashed lines above and below represent the S.E.M. B. Digital images of a vessel exposed to 4-AP (10 mM). In the presence of 4-AP some cells undergo small contractions (arrows in centre image). Movie 1 shows the contractions induced by 4-AP in this vessel.
Discussion
Most studies concerning the measurement of ionic currents in MVSM have used freshly isolated cells10, but there are several problems with this type of approach. In particular, it is often difficult to correlate the electrophysiology of the cells with arterioles of a known size, and it is likely that single cells change their properties when isolated from their tissue environment26. We therefore opted for a technique based upon direct patch-clamp recording from retinal MVSM cells which were still embedded within their parent arterioles. Although whole-cell patch clamp recordings have also been reported for coronary27, cerebral28 and choroidal19 arterioles, this technique has the drawback that endothelial cells are usually still present and may contaminate the current records originating from the MVSM. To avoid this problem, we devised a protocol whereby isolated retinal arterioles were anchored down in a recording bath and externally perfused with an enzyme cocktail. This resulted in a gradual delamination of the arterioles which could be monitored and stopped as soon as the endothelial and MVSM cell layers had fully separated (see Fig 1). The ability to monitor the dissociation of the cell layers proved extremely advantageous because slight over-digestion of the arterioles resulted in sustained contraction.
In the present study, we have identified and characterised an A-type potassium current in retinal MVSM cells. The features of the current, particularly the activation threshold, time constant for recovery from inactivation and high sensitivity to 4-AP, resemble those reported for A-type currents observed in other types of smooth muscle12, but there are some important differences. In particular, the voltage for half inactivation of the A-type current in retinal MVSM is ∼ 20-40 mV more positive than values previously reported. Furthermore, the current is partially suppressed by relatively low levels of TEA. These distinct characteristics may indicate that the molecular composition of the channels underlying the A-type current is different in retinal MVSM. Potassium channel α subunits with A-type properties are found in several potassium channel families including Shaker (KV 1.3, KV1.4 & KV1.7), Shaw (KV3.3 & KV3.4) and Shal (Kv4.1, Kv4.2, Kv4.3), and transcripts for most of these subunits have been detected in vascular smooth muscle29. Interestingly, none of these subunits, when expressed in heterologous expression systems11, mediate A-type currents with properties analogous to those presently described in retinal MVSM. This may be explained by the fact that KV channels within the same sub-family are able to form heteromultimeric channels which can exhibit hybrid biophysical and pharmacological properties11. Furthermore, in native cells, the presence and interaction of accessory β-subunits is also an important determinant of the kinetic features of the A-type current11,12. Considering the above, it is apparent that a whole host of distinct A-type K+ currents may exist and molecular-based studies are now warranted to identify the major components of the A-type current in retinal MVSM cells.
The functional importance of A-type currents in vascular smooth muscle depends upon the existence of sustained channel activity at physiological membrane potentials. The exact physiological function of A-type currents in vascular smooth muscle is controversial because in many cases the currents should be completely inactivated at the resting membrane potential12. Our experiments characterising the voltage-dependence of activation and inactivation in retinal MVSM cells suggest, however, that the voltage-window over which a steady state A-type current will persist, overlaps the resting membrane potential in this tissue. This was confirmed by testing the effects of 4-AP (which specifically blocks the A-type current in retinal arterioles, see Fig 4) in current-clamp mode. From an initial membrane voltage of -40 mV, 4-AP caused a small but measurable depolarisation. Some authors have previously suggested that the primary role of the A-type current in vascular smooth muscle is to suppress membrane excitability16,18. We have direct evidence for this in retinal MVSM, since application of 4-AP to current-clamped vessels not only caused membrane depolarisation, but also increased cell contractility. Thus, it seems probable that regulation of A-type channels at the molecular or functional level in retinal MVSM cells may have important implications for the control of local tissue perfusion in the retina.
To conclude, this is the first report to identify and characterise the A-type K+ current in retinal MVSM cells. We have begun to resolve the physiological significance this current, but further studies are now required to establish its molecular basis and its role in regulating retinal blood flow. Such studies may critically underpin future work aimed at providing a better understanding of retinal haemodynamic abnormalities in ocular diseases like diabetes.
Acknowledgements
We thank The Juvenile Diabetes Research Foundation (US), Fight for Sight (UK) and The Wellcome Trust for financial support. We thank Miss Claire Lagan for assistance with the vascular histology.
Footnotes
Scientific Section: PH
Disclosure for all authors: N
Reference List
- 1.Curtis TM, Tumelty J, Dawicki J, Scholfield CN, McGeown JG. Identification and Spatiotemporal Characterization of Spontaneous Ca2+ Sparks and Global Ca2+ Oscillations in Retinal Arteriolar Smooth Muscle Cells. Invest Ophthalmol Vis Sci. 2004;45:4409–14. doi: 10.1167/iovs.04-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye XD, Laties AM, Stone RA. Peptidergic innervation of the retinal vasculature and optic nerve head. Invest Ophthalmol Vis Sci. 1990;31:1731–7. [PubMed] [Google Scholar]
- 3.Delaey C, Van D,V. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 4.Guibert C, Beech DJ. Positive and negative coupling of the endothelin ETA receptor to Ca2+-permeable channels in rabbit cerebral cortex arterioles. J Physiol. 1999;514(Pt 3):843–56. doi: 10.1111/j.1469-7793.1999.843ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholfield CN, Curtis TM. Heterogeneity in cytosolic calcium regulation among different microvascular smooth muscle cells of the rat retina. Microvasc Res. 2000;59:233–42. doi: 10.1006/mvre.1999.2227. [DOI] [PubMed] [Google Scholar]
- 6.Curtis TM, Major EH, Trimble ER, Scholfield CN. Diabetes-induced activation of protein kinase C inhibits store-operated Ca2+ uptake in rat retinal microvascular smooth muscle. Diabetologia. 2003;46:1252–9. doi: 10.1007/s00125-003-1178-5. [DOI] [PubMed] [Google Scholar]
- 7.Gormley BA, Scholfield CN. Mechanism of calcium extrusion in microvascular smooth muscle in the rat retina. J Physiology. 2004:555P. [Google Scholar]
- 8.Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand. 1998;164:549–57. doi: 10.1046/j.1365-201X.1998.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 10.Jackson WF. Potassium channels and regulation of the microcirculation. Microcirculation. 1998;5:85–90. [PubMed] [Google Scholar]
- 11.Coetzee WA, Amarillo Y, Chiu J, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–85. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 12.Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- 13.Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. EMBO J. 2001;20:5715–24. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beech DJ, Bolton TB. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol. 1994;266:F325–F341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- 17.Halliday FC, Aaronson PI, Evans AM, Gurney AM. The pharmacological properties of K+ currents from rabbit isolated aortic smooth muscle cells. Br J Pharmacol. 1995;116:3139–48. doi: 10.1111/j.1476-5381.1995.tb15116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smirnov SV, Aaronson PI. Ca(2+)-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. J Physiol. 1992;457:431–54. doi: 10.1113/jphysiol.1992.sp019386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis TM, Scholfield CN. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J Physiol. 2001;532:609–23. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirst GD, Edwards FR, Gould DJ, Sandow SL, Hill CE. Electrical properties of iridial arterioles of the rat. Am J Physiol. 1997;273:H2465–H2472. doi: 10.1152/ajpheart.1997.273.5.H2465. [DOI] [PubMed] [Google Scholar]
- 21.Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–59. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knaus HG, McManus OB, Lee SH, et al. Tremorgenic indole alkaloids potently inhibit smooth muscle high-conductance calcium-activated potassium channels. Biochemistry. 1994;33:5819–28. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 23.Amberg GC, Baker SA, Koh SD, et al. Characterization of the A-type potassium current in murine gastric antrum. J Physiol. 2002;544:417–28. doi: 10.1113/jphysiol.2002.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashitani H, Windle A, Suzuki H. Neuroeffector transmission in arterioles of the guinea-pig choroid. J Physiol. 1998;510(Pt 1):209–23. doi: 10.1111/j.1469-7793.1998.209bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirst GD, Edwards FR. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989;69:546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- 26.Quinn K, Beech DJ. A method for direct patch-clamp recording from smooth muscle cells embedded in functional brain microvessels. Pflugers Arch. 1998;435:564–9. doi: 10.1007/s004240050553. [DOI] [PubMed] [Google Scholar]
- 27.Klieber HG, Daut J. A glibenclamide sensitive potassium conductance in terminal arterioles isolated from guinea pig heart. Cardiovasc Res. 1994;28:823–30. doi: 10.1093/cvr/28.6.823. [DOI] [PubMed] [Google Scholar]
- 28.Cheong A, Quinn K, Dedman AM, Beech DJ. Activation thresholds of K(V), BK andCl(Ca) channels in smooth muscle cells in pial precapillary arterioles. J Vasc Res. 2002;39:122–30. doi: 10.1159/000057761. [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K(+) channel genes in mesenteric artery smooth muscle cells. Am J Physiol. 1999;277:G1055–G1063. doi: 10.1152/ajpgi.1999.277.5.G1055. [DOI] [PubMed] [Google Scholar]