Abstract
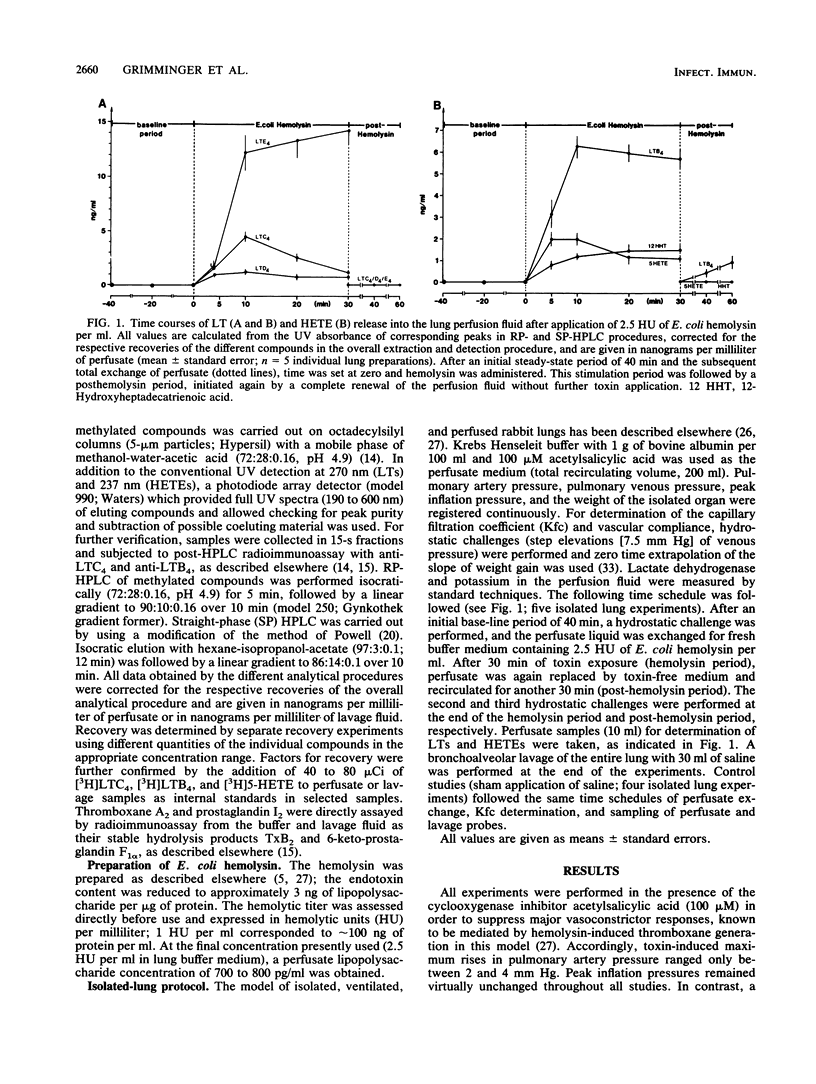
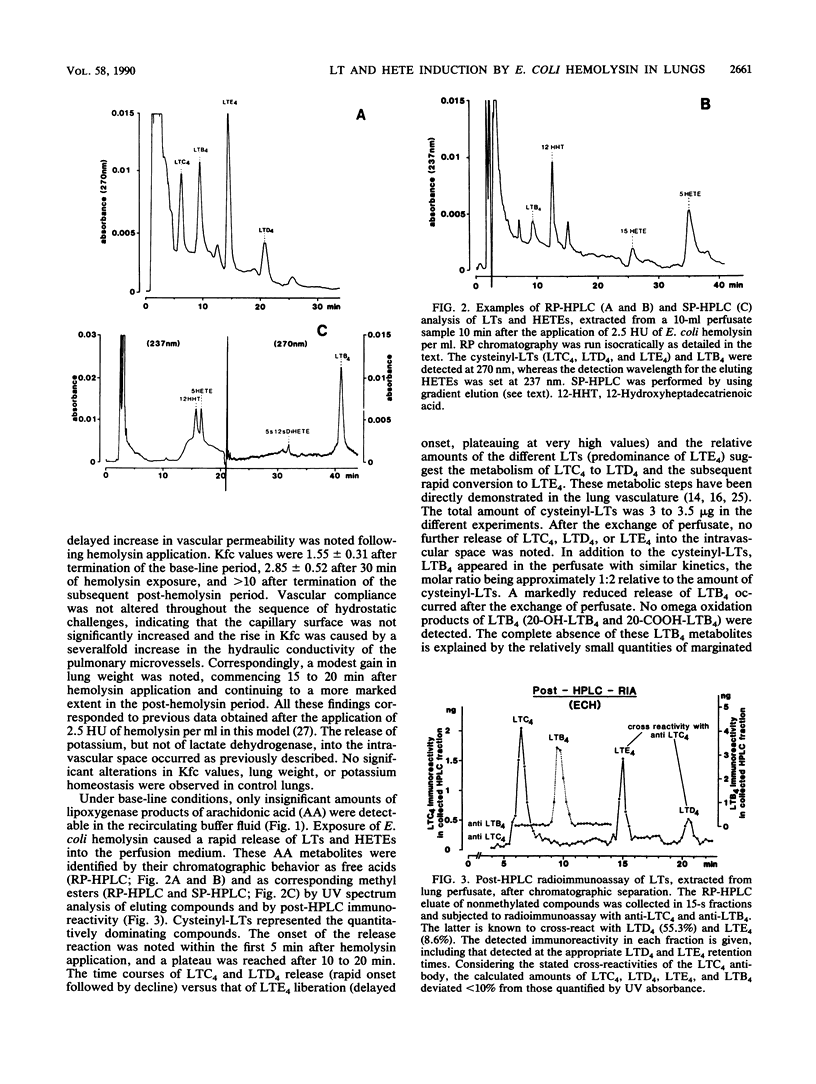
Low doses of Escherichia coli hemolysin cause thromboxane-mediated hypertension and vascular leakage in blood-free perfused rabbit lungs (W. Seeger, H. Walter, N. Suttorp, M. Muhly, and S. Bhakdi, J. Clin. Invest. 84:220-227, 1989). The recirculating buffer medium and bronchoalveolar lavage fluid from lungs exposed to hemolysin (2.5 hemolytic units per ml) in the presence of cyclooxygenase inhibitor were analyzed for leukotrienes (LTs) and hydroxyeicosatetraenoic acids (HETEs) by reverse-phase and straight-phase high-pressure liquid chromatographic techniques combined with UV spectrum analysis and post-high-pressure liquid chromatography radioimmunoassay. A rapid release of large amounts of cysteinyl-LTs and leukotriene B4 (LTB4) into the intravascular space was noted (total sum, approximately 4 to 5 micrograms). Similar quantities have hitherto been elicited only by high concentrations of the artificial calcium ionophore A 23187. Moreover, a marked liberation of 5-HETE and 12-hydroxyheptadecatrienoic acid into the buffer medium occurred, whereas LTB4 represented the predominant compound in the lavage fluid. The hemolysin-induced burst of LT and HETE generation preceded the onset of vascular leakage. The outstanding capacity of E. coli hemolysin to produce the liberation of potent lipid mediators is probably relevant to the pathways of vascular injury and amplification of inflammatory events during severe infection with hemolytic E. coli strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. K., Lamm W. J., Henderson W. R., Bolin R. W. Effect of leukotrienes B4, C4, and D4 on segmental pulmonary vascular pressures. J Appl Physiol (1985) 1989 Jan;66(1):458–464. doi: 10.1152/jappl.1989.66.1.458. [DOI] [PubMed] [Google Scholar]
- Andrews C. P., Coalson J. J., Smith J. D., Johanson W. G., Jr Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury. Chest. 1981 Sep;80(3):254–258. doi: 10.1378/chest.80.3.254. [DOI] [PubMed] [Google Scholar]
- Bertram T. A., Overby L. H., Danilowicz R., Eling T. E., Brody A. R. Pulmonary intravascular macrophages metabolize arachidonic acid in vitro. Comparison with alveolar macrophages. Am Rev Respir Dis. 1988 Oct;138(4):936–944. doi: 10.1164/ajrccm/138.4.936. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Greulich S., Muhly M., Eberspächer B., Becker H., Thiele A., Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989 Mar 1;169(3):737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Brigham K. L. Metabolites of arachidonic acid in experimental lung vascular injury. Fed Proc. 1985 Jan;44(1 Pt 1):43–45. [PubMed] [Google Scholar]
- Burhop K. E., Selig W. M., Malik A. B. Monohydroxyeicosatetraenoic acids (5-HETE and 15-HETE) induce pulmonary vasoconstriction and edema. Circ Res. 1988 Apr;62(4):687–698. doi: 10.1161/01.res.62.4.687. [DOI] [PubMed] [Google Scholar]
- Cavalieri S. J., Bohach G. A., Snyder I. S. Escherichia coli alpha-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984 Dec;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinmark S. J., Cannon P. J. Endothelial cell leukotriene C4 synthesis results from intercellular transfer of leukotriene A4 synthesized by polymorphonuclear leukocytes. J Biol Chem. 1986 Dec 15;261(35):16466–16472. [PubMed] [Google Scholar]
- Feuerstein N., Ramwell P. W. In vivo and in vitro effects of endotoxin on prostaglandin release from rat lung. Br J Pharmacol. 1981 Jun;73(2):511–516. doi: 10.1111/j.1476-5381.1981.tb10450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfstück R., Tschäpe H., Stein G., Kunath H., Bergner M., Wessel G. Virulence properties of Escherichia coli strains in patients with chronic pyelonephritis. Infection. 1986 May-Jun;14(3):145–150. doi: 10.1007/BF01643482. [DOI] [PubMed] [Google Scholar]
- Grimminger F., Becker G., Seeger W. High yield enzymatic conversion of intravascular leukotriene A4 in blood-free perfused lungs. J Immunol. 1988 Oct 1;141(7):2431–2436. [PubMed] [Google Scholar]
- Grimminger F., Menger M., Becker G., Seeger W. Potentiation of leukotriene production following sequestration of neutrophils in isolated lungs: indirect evidence for intercellular leukotriene A4 transfer. Blood. 1988 Nov;72(5):1687–1692. [PubMed] [Google Scholar]
- Harper T. W., Westcott J. Y., Voelkel N., Murphy R. C. Metabolism of leukotrienes B4 and C4 in the isolated perfused rat lung. J Biol Chem. 1984 Dec 10;259(23):14437–14440. [PubMed] [Google Scholar]
- Littner M. R., Kazmi G. M., Lott F. D. Inhibition of cyclooxygenase production does not prevent arachidonate from increasing extravascular lung water and albumin in an isolated dog lung. Prostaglandins Leukot Med. 1984 Jul;15(1):53–68. doi: 10.1016/0262-1746(84)90056-8. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Altman L. C., Albert R. K., Henderson W. R. Leukotriene B4 production by the human alveolar macrophage: a potential mechanism for amplifying inflammation in the lung. Am Rev Respir Dis. 1984 Jan;129(1):106–111. doi: 10.1164/arrd.1984.129.1.106. [DOI] [PubMed] [Google Scholar]
- Noonan T. C., Selig W. M., Burhop K. E., Burgess C. A., Malik A. B. Pulmonary microvascular response to LTB4: effects of perfusate composition. J Appl Physiol (1985) 1988 May;64(5):1989–1996. doi: 10.1152/jappl.1988.64.5.1989. [DOI] [PubMed] [Google Scholar]
- Powell W. S., Gravelle F. Metabolism of leukotriene B4 to dihydro and dihydro-oxo products by porcine leukocytes. J Biol Chem. 1989 Apr 5;264(10):5364–5369. [PubMed] [Google Scholar]
- Powell W. S. Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes. J Biol Chem. 1984 Mar 10;259(5):3082–3089. [PubMed] [Google Scholar]
- Rinaldo J. E., Rogers R. M. Adult respiratory distress syndrome. N Engl J Med. 1986 Aug 28;315(9):578–580. doi: 10.1056/NEJM198608283150909. [DOI] [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Seeger W., Menger M., Walmrath D., Becker G., Grimminger F., Neuhof H. Arachidonic acid lipoxygenase pathways and increased vascular permeability in isolated rabbit lungs. Am Rev Respir Dis. 1987 Oct;136(4):964–972. doi: 10.1164/ajrccm/136.4.964. [DOI] [PubMed] [Google Scholar]
- Seeger W., Walmrath D., Menger M., Neuhof H. Increased lung vascular permeability after arachidonic acid and hydrostatic challenge. J Appl Physiol (1985) 1986 Nov;61(5):1781–1789. doi: 10.1152/jappl.1986.61.5.1781. [DOI] [PubMed] [Google Scholar]
- Seeger W., Walter H., Suttorp N., Muhly M., Bhakdi S. Thromboxane-mediated hypertension and vascular leakage evoked by low doses of Escherichia coli hemolysin in rabbit lungs. J Clin Invest. 1989 Jul;84(1):220–227. doi: 10.1172/JCI114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger W., Wolf H., Stähler G., Neuhof H., Róka L. Increased pulmonary vascular resistance and permeability due to arachidonate metabolism in isolated rabbit lungs. Prostaglandins. 1982 Feb;23(2):157–173. doi: 10.1016/0090-6980(82)90043-0. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Habben E. Effect of staphylococcal alpha-toxin on intracellular Ca2+ in polymorphonuclear leukocytes. Infect Immun. 1988 Sep;56(9):2228–2234. doi: 10.1128/iai.56.9.2228-2234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Dewein E., Bhakdi S., Roka L. Staphylococcal alpha-toxin-induced PGI2 production in endothelial cells: role of calcium. Am J Physiol. 1985 Jan;248(1 Pt 1):C127–C134. doi: 10.1152/ajpcell.1985.248.1.C127. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Uhl J., Lutz F., Roka L. Pseudomonas aeruginosa cytotoxin stimulates prostacyclin production in cultured pulmonary artery endothelial cells: membrane attack and calcium influx. J Cell Physiol. 1985 Apr;123(1):64–72. doi: 10.1002/jcp.1041230111. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Seeger W., Zucker-Reimann J., Roka L., Bhakdi S. Mechanism of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun. 1987 Jan;55(1):104–110. doi: 10.1128/iai.55.1.104-110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Falkow S. Characterization of Escherichia coli hemolysins conferring quantitative differences in virulence. Infect Immun. 1984 Jan;43(1):156–160. doi: 10.1128/iai.43.1.156-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott J. Y., McDonnell T. J., Bostwick P., Voelkel N. F. Eicosanoid production in isolated perfused lungs stimulated by calcium ionophore A23187. Am Rev Respir Dis. 1988 Oct;138(4):895–900. doi: 10.1164/ajrccm/138.4.895. [DOI] [PubMed] [Google Scholar]
- Winn R., Nickelson S., Rice C. L. Fluid filtration coefficient of isolated goat lungs was unchanged by endotoxin. J Appl Physiol (1985) 1988 Jun;64(6):2463–2467. doi: 10.1152/jappl.1988.64.6.2463. [DOI] [PubMed] [Google Scholar]


