Abstract
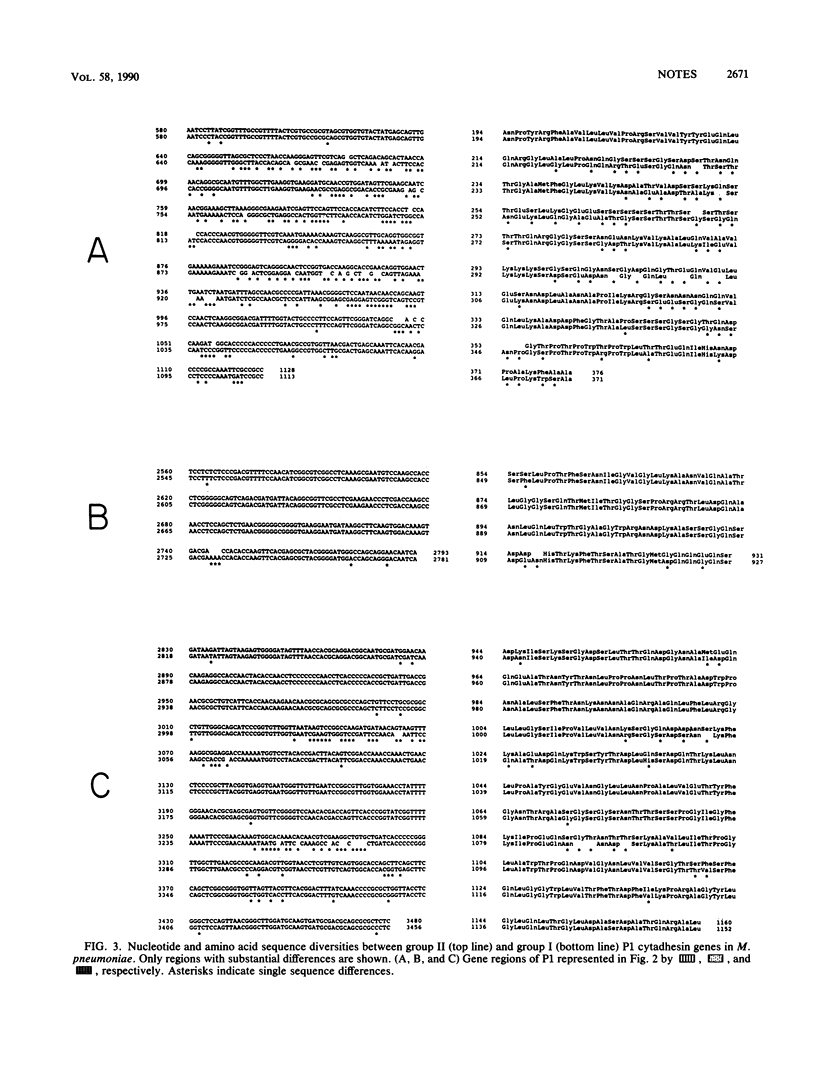
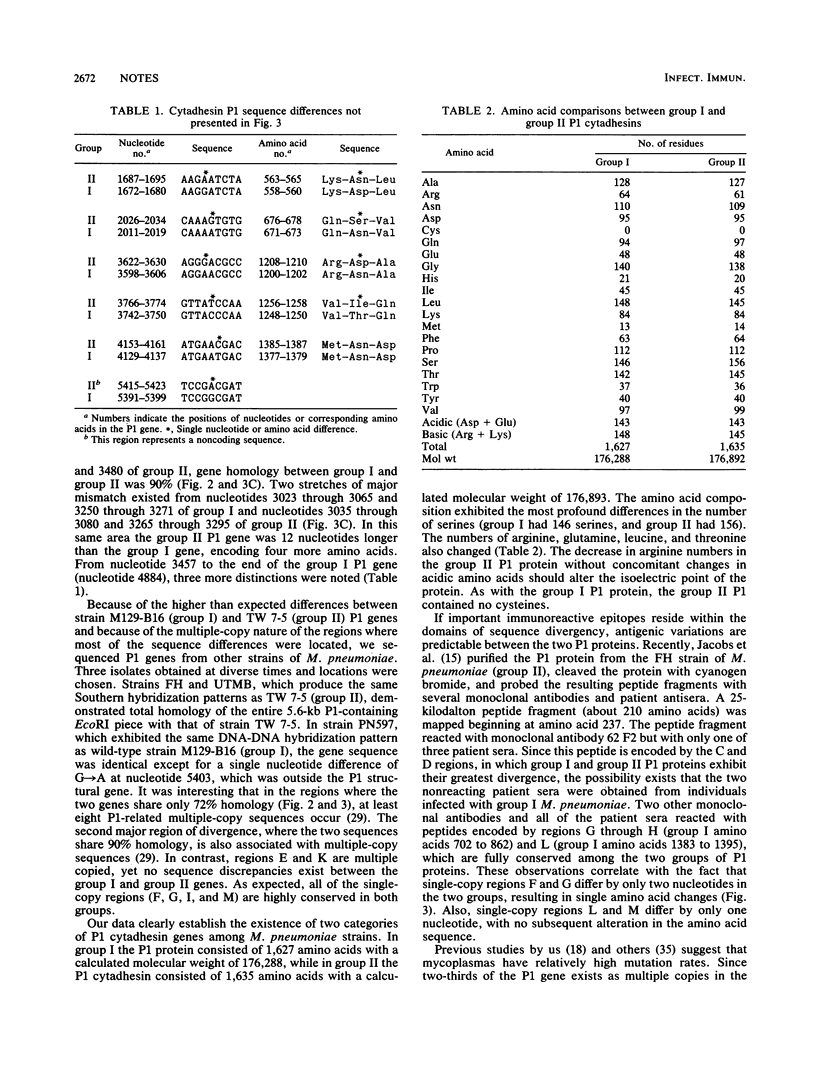
The Mycoplasma pneumoniae cytadhesin P1 genes from two groups of clinical isolates that display restriction fragment length polymorphisms were cloned and sequenced. Within each group the nucleotide sequences were identical, but two major differences were detected between the groups. These two stretches of sequence divergence were located in multiple-copy regions of the P1 gene and resulted in considerable amino acid changes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Banai M., Kahane I. Sialic acid residues mediate Mycoplasma pneumoniae attachment to human and sheep erythrocytes. Infect Immun. 1982 Oct;38(1):389–391. doi: 10.1128/iai.38.1.389-391.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Dallo S. F., Tully J. G., Rose D. L. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988 Nov;26(11):2266–2269. doi: 10.1128/jcm.26.11.2266-2269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Horton J. R., Su C. J., Baseman J. B. Restriction fragment length polymorphism in the cytadhesin P1 gene of human clinical isolates of Mycoplasma pneumoniae. Infect Immun. 1990 Jun;58(6):2017–2020. doi: 10.1128/iai.58.6.2017-2020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Su C. J., Horton J. R., Baseman J. B. Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 1988 Feb 1;167(2):718–723. doi: 10.1084/jem.167.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Cochran S., Lisse J., Buck G., DiNuzzo A. R., Weber T., Reinarz J. A. Isolation of Mycoplasma pneumoniae from synovial fluid samples in a patient with pneumonia and polyarthritis. Arch Intern Med. 1988 Apr;148(4):969–970. [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., McMahan R., Mansy A. M., Grayston J. T. Mycoplasma pneumoniae pneumonia in an urban area. Five years of surveillance. JAMA. 1970 Nov 30;214(9):1666–1672. [PubMed] [Google Scholar]
- GRAYSTON J. T., ALEXANDER E. R., KENNY G. E., CLARKE E. R., FREMONT J. C., MACCOLL W. A. MYCOPLASMA PNEUMONIAE INFECTIONS. CLINICAL AND EPIDEMIOLOGIC STUDIES. JAMA. 1965 Feb 1;191:369–374. doi: 10.1001/jama.1965.03080050015004. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E., Gerstenecker B., Mader B., Huang C. H., Hu P. C., Halter R., Bredt W. Binding sites of attachment-inhibiting monoclonal antibodies and antibodies from patients on peptide fragments of the Mycoplasma pneumoniae adhesin. Infect Immun. 1989 Mar;57(3):685–688. doi: 10.1128/iai.57.3.685-688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane I., Pnini S., Banai M., Baseman J. B., Cassell G. H., Bredt W. Attachment of mycoplasmas to erythrocytes: a model to study mycoplasma attachment to the epithelium of the host respiratory tract. Isr J Med Sci. 1981 Jul;17(7):589–592. [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr The interrelationship of virulence, cytadsorption, and peroxide formation in Mycoplasma pneumoniae. Proc Soc Exp Biol Med. 1969 Sep;131(4):1163–1167. doi: 10.3181/00379727-131-34061. [DOI] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Childs R. A., Paulson J. C., Rogers G. N., Scudder P. R., Michalski J. C., Hounsell E. F., Taylor-Robinson D., Feizi T. Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type. Nature. 1984 Feb 9;307(5951):560–563. doi: 10.1038/307560a0. [DOI] [PubMed] [Google Scholar]
- Loomes L. M., Uemura K., Feizi T. Interaction of Mycoplasma pneumoniae with erythrocyte glycolipids of I and i antigen types. Infect Immun. 1985 Jan;47(1):15–20. doi: 10.1128/iai.47.1.15-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J. T., Simon M. I., Barbour A. G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985 Jun;41(2):403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Hagblom P., Seifert H. S., So M. Antigenic variation of gonococcal pilus involves assembly of separated silent gene segments. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2177–2181. doi: 10.1073/pnas.83.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Baseman J. B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988 Dec;56(12):3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Koomey J. M. Gene conversion variations generate structurally distinct pilin polypeptides in Neisseria gonorrhoeae. J Exp Med. 1987 Apr 1;165(4):1016–1025. doi: 10.1084/jem.165.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Rose D. L., Whitcomb R. F., Wenzel R. P. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis. 1979 Apr;139(4):478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]
- Vu A. C., Foy H. M., Cartwright F. D., Kenny G. E. The principal protein antigens of isolates of Mycoplasma pneumoniae as measured by levels of immunoglobulin G in human serum are stable in strains collected over a 10-year period. Infect Immun. 1987 Aug;55(8):1830–1836. doi: 10.1128/iai.55.8.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R. P., Craven R. B., Davies J. A., Hendley J. O., Hamory B. H., Gwaltney J. M., Jr Field trial of an inactivated Mycoplasma pneumoniae vaccine. I. Vaccine efficacy. J Infect Dis. 1976 Dec;134(6):571–576. doi: 10.1093/infdis/134.6.571. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]


