Abstract
Integrin-linked kinase (ILK) and its binding partners α-parvin, β-parvin, Mig-2 and Migfilin are important components of the cell–matrix adhesions implicated in cell motility, growth, survival and ultimately carcinogenesis. Herein, we investigated immunohistochemically the expression of these molecules in cartilaginous neoplasms and explored their involvement in chondrosarcoma pathobiology and behaviour. Our analyses revealed that ILK, α-parvin, β-parvin and Mig-2 are expressed in the majority of chondrosarcomas but in a small proportion of enchondromas, implying that these proteins might have a role in the development and progression of chondrogenic neoplasms. Moreover, our findings highlight the possibilities that ILK might serve as biological marker that could accurately predict a highgrade tumour and that Mig-2 may function as a promising prognostic indicator of high-risk patients.
Keywords: Chondrosarcoma, ILK, Migfilin, Mig-2, Parvins, Prognosis, Survival
1. Introduction
Cell–extracellular matrix (ECM) adhesions play a crucial role in the regulation of most vital cellular processes. Integrinlinked kinase (ILK), an important component of cell–ECM adhesions, is a widely expressed Ser/Thr kinase that interacts with the cytoplasmic domains of β1 and β3 integrins, thus linking the ECM with the intracellular compartment. Interestingly, ILK serves a dual role at the cell–ECM adhesion sites; first, it acts as an adaptor protein forming a stable ternary complex that connects the ECM with actin cytoskeleton and second, it acts as a Ser/Thr kinase transmitting signals to the inner part of the cell.1,2 In vitro experiments have demonstrated that ILK activity is regulated in a phosphoinositide 3- kinase (PI3-K)-dependant fashion either by integrin clustering or by growth factors and chemokines.2,3 This results in the phosphorylation and hence activation of multiple signal transduction pathways such as AKT/protein kinase B (PKB), glycogen synthase kinase-3β (GSK-3β), PK that regulate important cellular functions including growth and survival, cell-cycle progression, cell motility, vascular development, tumour invasion, migration and angiogenesis.4–6
As an adaptor protein, ILK has the ability to bind to the particularly interesting new Cys–His-rich protein (PINCH) and parvins, all of which are critical components of the integrin-mediated signalling, forming a stable ternary protein complex at cell–ECM adhesion sites known as PIP complex. 2,6,7 The mammalian parvin family is composed of α-, β- and γ-parvin, which are encoded by three different genes. Human α- and β-parvins are closely related in terms of structure displaying 74% identity and 85% homology.8 Notably, these proteins are extensively expressed in human tissues, whereas the distribution of γ-parvin is more restricted.9
Further adding to the complexity of the matter, ILK is also known to interact with other cell–ECM adhesion proteins such as mitogen inducible gene-2 (Mig-2). Mig-2 is critical for a number of fundamental cellular processes but its role in carcinogenesis has yet to be defined. Mig-2 binds to Migfilin, another cell–ECM adhesion protein, which in turn associates with Filamin, linking the ECM to the actin cytoskeleton machinery.10,11
During the last few years a considerable volume of studies have been conducted to shed light upon the role of ILK in the pathobiology of human neoplasia. Indeed, immunohistochemical and biochemical data have shown that increased ILK expression is implicated in the pathogenesis and progression of several human malignancies, including melanoma, Ewing’s sarcoma, and colon, gastric, lung, prostate, ovarian and breast cancers.12–15 Interestingly, in vitro experiments in cell lines have demonstrated important roles for the ILK interactors in cell adhesion, spreading, motility, differentiation and survival.16 Nonetheless, the expression of these molecules in human tumours and their implication in tumorigenesis has not been studied extensively.
In this study we assessed the expression of ILK and its binding partners, α-parvin, β-parvin and Mig-2, as well as the expression of Migfilin in a well-characterised panel of human primary, central chondrosarcomas (CHS) of all grades, enchondromas (ECH) and normal cartilage. Moreover, we explored their role in the pathogenesis and progression of cartilaginous neoplasms and assessed their potential as possible biomarkers for the prediction of cartilage tumour grade. Finally, we examined these proteins as possible prognostic factors for the survival of CHS patients.
2. Patients and methods
2.1. Patients
We used formalin-fixed, paraffin-embedded tissue obtained from 60 primary, central (conventional) CHS and 25 ECH patients that were diagnosed at the University Hospitals of Patras and Ioannina, Greece as well at the ‘Agios Savvas’ and ‘Agia Olga’ Oncology Institutes, Athens, Greece, between 1993 and 2005. The study protocol was approved by the Ethics Committee of the University of Athens Medical School. Follow-up data were available for 40 of 60 patients (66.7%). The mean follow-up period was 67.9 months (STD = 40.9, range = 2–180 months). During this period of time 11 of 24 (45.8%) patients with high-grade (HG) and 3 of 16 (18.75%) patients with low-grade (LG) CHS died of their disease.
With respect to CHS patients, 34 were male and 26 were female (mean age 54 year, range 21–85 year). In 32 cases (54%) the tumour was developed in the limbs of the patients (femur: 25 cases; humerus: 7 cases). In the remaining 28 patients (34%), the neoplasm was located at the axial skeleton (pelvis: 22 cases; shoulder: 7 cases). There were 20 (33.3%) grade 1, 29 (48.3%) grade 2 and 11 (18.3%) grade 3 tumours. Twenty-three of the CHS of our series (39%) were smaller than 8 cm, whereas the remaining 37 tumours (61%) were bigger or equal to 8 cm.
The determination of the CHS grade was based upon established, widely accepted microscopic criteria.17,18 Specifically, the tumour architecture, cellularity and necrosis as well as the presence of specific cellular and nuclear features namely hyperchromasia, mitoses, nuclear enlargement and binucleation were assessed. Grade 1 CHS do not have metastatic potential and the 5-year survival for the patients with this tumour is 89%; on the contrary, grades 2 and 3 CHS metastasise in 10–33% and 70% of the cases, respectively, and the combined group of patients has a 5-year survival of 53%.18 In agreement with the aforementioned data, we considered grade 1 CHS as LG, whereas grades 2 and 3 as HG tumours.
Regarding the ECH patients, 11 were male and 14 female (mean age 52 years, range 31–67 years). All ECH were located at the extremities (humerus: 12; femur: 9; tibia: 4). Twenty normal hyaline cartilage specimens were obtained from autopsy material.
The best preserved sections from each case were selected for immunohistochemistry. Sections from areas with extensive myxoid changes and necrosis were excluded from the analysis.
2.2. Antibodies and immunohistochemistry
We generated IgG mouse monoclonal anti-ILK, anti-α-parvin, anti-β-parvin, anti-Mig-2 and anti-Migfilin antibodies at our Laboratory as previously reported.10
Following deparaffinisation, the classic biotin–streptavidin –peroxidase assay was performed on 4-μm thick, formalin-fixed, paraffin-embedded sections, as previously described.19 The following purified monoclonal antibodies were used: anti-ILK (concentration: 20 μg/ml, dilution: 1/100), anti-α-parvin (concentration: 7 μg/ml, dilution: 1/50), anti-β-parvin (concentration: 12.4 μg/ml, dilution: 1/100), anti-Mig-2 (concentration: 12.46 μg/ml, dilution: 1/50) and anti-Migfilin (concentration: 1.4 μg/ml, dilution: 1/70). Normal skeletal muscle was used as positive control for all antibodies tested. Vessel endothelial cells served as internal positive control for Migfilin staining. In negative control slides, the primary antibody was substituted with 1% TBS.
A minimum of 500 cells were examined in each specimen. Stain intensity and proportion of immunopositive cells were assessed by light microscopy and evaluated independently by two investigators (D.J.P. and A.G.P.). For all proteins, immunohistochemical staining was graded on a scale of 0–3+, according to the following assumption: 0: no immunoreactivity; 1+: mild immunoreactivity, 1–33% positive tumour cells; 2+: moderate immunoreactivity, 33–66% positive tumour cells; 3+: strong immunoreactivity, 67–100% positive tumour cells.22 In a very small number of cases inter-observer variation was noticed. These cases were re-examined and re-evaluated by both D.J.P. and A.G.P. and a consensus score was placed.
2.3. Statistical analyses
Mann-Whitney test was employed to compare non-parametric variable scores between HG and LG CHS, as well as between LG CHS and ECH. The strength of association amongst nominal variables was assessed by Kendall’s τ-test. Tumour grade was modelled using a binary logistic regression analysis; graded levels of protein expression were entered in the model as categorical predictors. Expression levels of all proteins were recoded from the four-level scale (0–3+) into a two-level scale (‘low/high’ expression), with a cutoff value for immunopositivity at 33%. Analyses for overall survival were evaluated using the Kaplan–Meier method, and different subgroups were compared using the log-rank test. Overall survival was calculated fromthe date of diagnostic biopsy until death because of the disease. Multivariate survival analysis was performed with the Cox regression model. All differences were considered statistically significant if P < 0.05. P-values were two-tailed. All statistical analyses were performed using the SPSS 13.0 for Windows (version 13.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Expression of ILK, α-parvin and β-parvin
Fifty six of the 60 (93.3%) CHS of all grades and 9 of 25 (36.0%) ECH exhibited primarily cytoplasmic immunoreactivity for ILK (Table 1). ILK expression pattern was similar between all cartilage tumours examined (Figs. 1A and B, 2B–D). α-parvin was expressed in 57/60 (95.0%) and β-parvin in 49/60 (81.7%) of the examined sarcomas. With respect to ECH, α-parvin immunopositivity was observed in 18 (72.0%) and β-parvin in 10 (40.0%) of 25 neoplasms, respectively. Notably, the localisation of parvinswas also mainly cytoplasmic (Fig. 1C and D). There was no variation in the immunoexpression fashion in different grades of CHS and ECH. Normal cartilage cells were not immunoreactive for the aforementioned molecules. Interestingly, the expression levels of ILK and α-parvin were significantly and positively correlated to each other (Kendall’s τ = 0.424, P = 0.01). No significant association was observed between the cellular levels of ILK and β-parvin (Kendall’s τ = 0.208, P = 0.12), as well as between the expression levels of α- and β-parvin (Kendall’s τ = 0.142, P = 0.853) (Fig. 2).
Table 1.
Immunohistochemical expression of the adhesion molecules ILK, α-parvin, β-parvin and Mig-2 in human chondrosarcomas
| ILK
|
α-Parvin
|
β-Parvin
|
Mig-2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intensity of IHC | G1 CHS | G2 CHS | G3 CHS | G1 CHS | G2 CHS | G3 CHS | G1 CHS | G2 CHS | G3 CHS | G1 CHS | G2 CHS | G3 CHS |
| 0 | 3 (15%) | 1 (4%) | 0 (0%) | 3 (15%) | 0 (0%) | 0 (100%) | 7 (35%) | 2 (8%) | 2 (13.3%) | 6 (30%) | 3 (12%) | 0 (0%) |
| 1+ | 10 (50%) | 1 (4%) | 0 (0%) | 5 (25%) | 4 (16%) | 3 (20%) | 4 (20%) | 14 (56%) | 1 (6.7%) | 5 (25%) | 4 (16%) | 1 (66%) |
| 2+ | 6 (30%) | 13 (52%) | 0 (0%) | 8 (40%) | 13 (52%) | 3 (20%) | 8 (40%) | 6 (24%) | 8 (53.3%) | 9 (45%) | 13 (52%) | 3 (20%) |
| 3+ | 1 (5%) | 10 (4%) | 15 (100%) | 4 (20%) | 8 (32%) | 9 (60%) | 1 (5%) | 3 (12%) | 4 (26.7%) | 0 (0%) | 5 (20%) | 11 (73.3%) |
| Positive cases | 17 (85%) | 24 (96%) | 15 (100%) | 17 (85%) | 25 (100%) | 15 (100%) | 13 (65%) | 23 (92%) | 13 (86.7%) | 14 (70%) | 22 (88%) | 15 (100%) |
| Total | 20 (100%) | 25 (100%) | 15 (100%) | 20 (100%) | 25 (100%) | 15 (100%) | 20 (100%) | 25 (100%) | 15 (100%) | 20 (100%) | 25 (100%) | 15 (100%) |
IHC, immunohistochemistry; ILK, integrin-linked kinase; CHS, chondrosarcoma; G, grade. G1 and G2 CHS = low-grade tumours; G3 CHS = high-grade tumours.
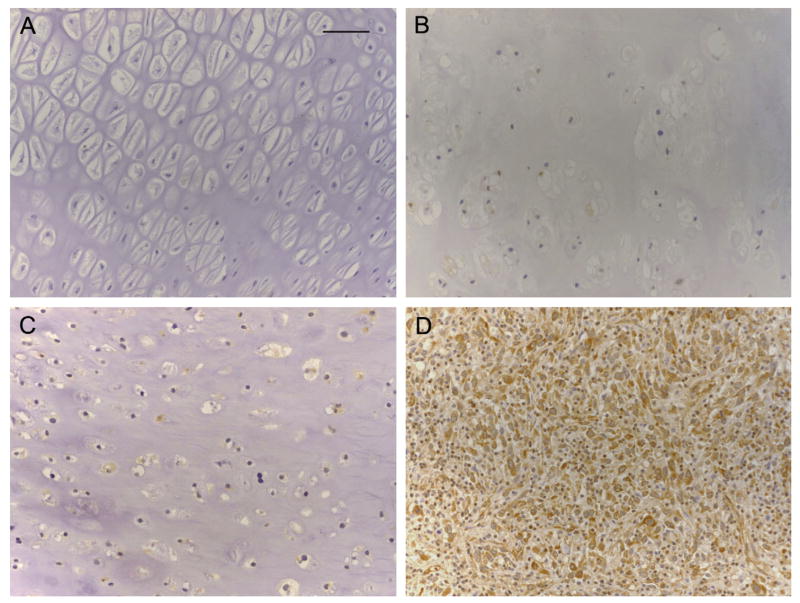
Fig. 1.
(A–E). Immunohistochemical profile of ILK, α-parvin, β-parvin and Mig-2 in HG CHS (original magnifications ×20, ×40, ×20, ×20, respectively). (F) Migfilin displayed no immunoreactivity. Nonetheless, the endothelial cells showed intense immunopositivity for this adhesion protein (original magnification ×20). Scale bar = 50 μm.
Fig. 2.
(A) ILK is not expressed in normal hyaline cartilage. (B) Weak positivity for ILK in an ECH. (C) LG CHS displaying moderate ILK immunoexpression. (D) Malignant cells of a HG CHS exhibiting strong, cytoplasmic ILK immunostaining (A–D, original magnification ×20). Scale bar = 50 μm.
3.2. Expression of Mig-2 and Migfilin
Mig-2 was expressed in 51/60 CHS (85%) but only in 4/25 (16%) ECH (Table 1). In the vast majority of the examined cells Mig-2 immunolocalisation was almost exclusively cytoplasmic (Fig. 1E). The immunohistochemical profile of Mig-2 was very similar in all the cases examined. Normal cartilage specimens were negative for this protein. Notably, the expression levels of Mig-2 were strongly correlated to the cellular levels of ILK (Kendall’s τ = 0.490, P < 0.0001) and α-parvin (Kendall’s τ = 0.384, P < 0.0001). On the contrary, no association was revealed between Mig-2 and β-parvin staining levels (Kendall’s τ = 0.025, P = 0.853).
In all the CHS and ECH cases the tumour cells were negative for Migfilin. Nonetheless, the endothelial cells exhibited strong immunopositivity for Migfilin in both neoplastic and normal cartilage (Fig. 1F).
3.3. Correlation of ILK and its binding partners, α-parvin, β-parvin and Mig-2, with tumour grade in human chondrogenic neoplasms
The expression levels of ILK and Mig-2 were significantly higher in HG in comparison to LG CHS and in LG CHS compared to ECH (Mann-Whitney test, P < 0.005 for all). Applying binary logistic regression tests, we assessed the potential role of ILK and Mig-2 as tumour grade predictors. Our analysis revealed that ILK expression status displayed the strongest association with tumour grade. Specifically, ILK cellular levels could predict a HG CHS with a positive predictive value 95%, negative predictive value 65% and an overall accuracy 85%.
3.4. Survival analysis
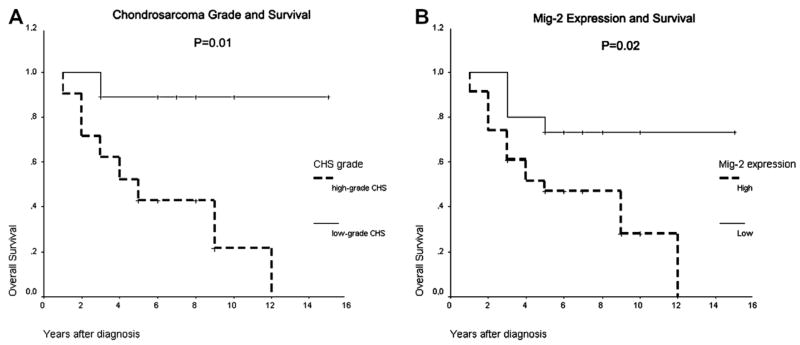
Univariate analysis revealed that advanced tumour grade and higher levels of Mig-2 expression correlated with worse overall survival (log-rank P = 0.01 and 0.02, respectively) (Fig. 3A and B). ILK expression revealed a strong tendency for association with poor prognosis, but the observed correlation (P = 0.06) did not reach the formal level of significance. α-parvin and β-parvin expression, as well as patient gender, tumour location and size had no impact on survival. Cox multivariate analysis of survival revealed that tumour grade was the single, independent prognostic factor for poor outcome (log-rank P = 0.04).
Fig. 3.
(A) Kaplan–Meier curve for the overall survival of the patients with high- and low-grade CHS. The two curves are significantly different (P = 0.01). (B) Kaplan–Meier curve for the overall survival of the CHS patients, according to the expression of Mig-2. The two curves are significantly different (P = 0.02).
4. Discussion
Over the past few years, reports froma number of laboratories have provided evidence suggesting that ILK is a pivotal regulator of numerous aspects of cell morphology and biological behaviour, and its deregulation has been implicated in tumourigenesis. Accordingly, several in vitro studies have documented that ILK overexpression suppresses apoptosis, increases resistance to cell death in suspension (anoikis) and promotes cell survival primarily through AKT/PKB and GSK-3β phosphorylation.20,21 In addition, ILK overexpression or constitutive activation contributes to cell-cycle progression, whereas suppression of ILK function results in inhibition of cyclin D1 expression and G1/S cell-cycle arrest.22 Therefore, up-regulation of this protein has been related to the pathobiology of several human cancers.12–15 Although knock-out experiments on mice have shown that ILK is implicated in chondrocyte growth and shape regulation,23 the expression of ILK in cartilaginous tumours and its implication in their pathogenesis has not been investigated.
In this study we detected ILK expression in the majority of the cartilaginous tumours evaluated, but not in normal chondrocytes. Notably, statistical analysis of our results uncovered that ILK expression correlated significantly and positively with tumour grade. This is supported by similar findings in other human malignancies, such as colon cancer and melanoma, where increased ILK expression was significantly linked to advanced tumour grade and stage.13,14 Collectively, our results provide evidence that ILK may have a key role in the pathogenesis of human cartilaginous sarcomas and, most importantly, the extent of its expression is probably associated with the development and progression of these malignancies.
Another finding of our study is that the binding partners of ILK, α-parvin and β-parvin, were readily expressed in the majority of the examined tumours, whereas normal cartilage did not express these proteins in detectable levels. This indicates that parvins may be involved in the malignant transformation of chondroblasts. Even though parvins have been shown to participate in actin cytoskeleton regulation and cell survival, very few studies have attempted to investigate their role in human disease. Cell culture experiments, however, have revealed marked down-regulation of β-parvin in advanced epithelial breast cancer.24 This finding is in contrast to our results of increased β-parvin expression in HG CHS, but the discrepancy may reflect a different role of β-parvin in the pathobiology of tumours derived from different precursor cells. Additional studies in large number of tumours are needed in order to determine the exact role of β-parvin in human oncogenesis.
Several in vitro studies have documented that α- and β-parvin display similar, Cys–His domain-dependant binding to the COOH-terminal region of ILK and therefore, they act antagonistically in order to accomplish exclusive ILK binding.25 This competitive behaviour responds to the distinct functions of these two adhesion molecules. Specifically, although α-parvin protects cell from apoptosis, inducing the activation of AKT/PKB-mediated survival signalling, experiments in HeLa cells have documented that β-parvin induces the apoptotic pathways. 8 In our study, ILK and parvins were found to be coimmunolocalised in similar cellular compartments. Importantly, the cellular levels of ILK and α-parvin displayed very strong association, whereas the degree of their expression was significantly related to the histological grade of the examined neoplasms. The parallel up-regulation of ILK and α-parvin may be of importance, since it most likely indicates that ILK and α-parvin functionally interact in the cytoplasm of neoplastic cells forming a protein complex that appears to be involved in the pathobiology of human CHS.
With regard to Mig-2, another ILK-interacting cell–ECM adhesion protein, our study revealed that it was highly expressed in the majority of the CHS but only in a small fraction of the ECH and not at all in normal cartilage. Moreover, our results demonstrated that the cellular levels of Mig-2 were significantly increased in HG, compared with LG CHS and ECH. Remarkably, Mig-2 immunolocalisation paralleled the localisation of ILK and α-parvin and its expression levels were robustly and positively associated with the cellular levels of these two molecules. Taken together, our findings imply that Mig-2 might be involved in CHS pathobiology, possibly through interaction with ILK and α-parvin.
Migfilin is a Mig-2-binding protein encountered at cell– ECM adhesions in fibroblasts and at both cell–ECM and cell– cell adhesions in epithelial and endothelial cells10,26 In vitro experiments have shown that Migfilin is crucial for cell adhesion and spreading, and it regulates cell shape and migration and strengthens the cell–cell adhesions between neighbouring cells.11 Interestingly, recent data show that Migfilin is expressed in human leiomyomas and leiomyosarcomas and that its expression levels are associated with higher tumour grades.27 In this study however, Migfilin could not be detected in any of the cartilaginous neoplasms examined. This may indicate that Migfilin has different functions in these two different categories of mesenchymal tumours. Remarkably, however, in both studies endothelial cells displayed strong Migfilin immunopositivity. It would be of great interest to further test the significance of this protein as a potential novel endothelial marker.
We also assessed the cellular levels of ILK, α-parvin, β-parvin and Mig-2 as biological markers that could aid in the discrimination between HG and LG CHS. Binary logistic regression analysis revealed that elevated immunoexpression of ILK could accurately predict HG tumours. This is potentially a notable finding. Recent therapeutic protocols suggest that a considerable volume of extremity LG CHS can be treated with local curettage and cryosurgery, whilst HG CHS patients should undergo aggressive surgical procedures.28,29 Consequently, precise distinction between these two tumour categories might contribute to an individualised assessment of prognosis and therapeutic intervention. In our study however, limitations of statistical analyses related to the size of our sample did not allow extrapolation of this conclusion beyond this series.
In line with previous data, we found that higher tumour grade was the single independent parameter related to poor patient outcome.18,30 Univariate analysis revealed that Mig-2 expression could function as a prognostic factor for the overall survival of the CHS patients of our sample. However, because of the limited number of our series and the retrospective nature of our study, the importance of Mig-2 as biomarker for tumour prognosis should be drawn with caution. Additional, multicentric, preferentially prospective studies are required to determine whether this molecule could be helpful in patient evaluation in an individual basis.
In conclusion, our study provides novel evidence that ILK, α-parvin and Mig-2 are expressed in human cartilaginous tumours and, most importantly, that these proteins may have a possible —yet unidentified— role in the development and progression of these neoplasms. Furthermore, it underscores the potential function of ILK as molecular marker for the prediction of HG tumours and highlights the possibility that Mig- 2 may act as promising prognostic factor for poor survival. If the participation of the aforementioned adhesion molecules in the pathobiology of CHS proves to be essential, treatment with small-molecule drugs that could selectively disrupt the interaction between ILK and α-parvin at focal adhesions might have great benefit in the treatment of CHS patients.
Acknowledgments
This study was supported in part by NIH Grants GM65188 and DK54639 to C.W.
Footnotes
Conflict of interest statement
None declared.
References
- 1.Brakebusch C, Fässler R. The integrin-actin connection, an eternal love affair. EMBO J. 2003;22:2324–33. doi: 10.1093/emboj/cdg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legate KR, Montañez E, Kudlacek O, Fässler R. ILK, PINCH and parvin: the tIPP of integrin signaling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 3.Khwaja A, Rodriquez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan C, Cruet-Hennequart S, Troussard A, et al. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko Y, Kitazato K, Basaki Y. Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor. J Cell Sci. 2004;26:407–15. doi: 10.1242/jcs.00871. [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505–10. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Chen K, Tu Y, et al. Assembly of the PINCH-ILK-CHILKBP complex precedes and is essential for localization of each component to cell–matrix adhesion sites. J Cell Sci. 2002;115:4777–86. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 8.Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci. 2006;63:25–35. doi: 10.1007/s00018-005-5355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenbaum E, Olski TM, Noegel AA. Genomic organization and expression profile of the parvin family of focal adhesion proteins in mice and humans. Gene. 2001;279:69–79. doi: 10.1016/s0378-1119(01)00743-0. [DOI] [PubMed] [Google Scholar]
- 10.Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 11.Gkretsi V, Zhang Y, Tu Y, et al. Physical and functional association of migfilin with cell–cell adhesions. J Cell Sci. 2005;118:697–710. doi: 10.1242/jcs.01638. [DOI] [PubMed] [Google Scholar]
- 12.Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–84. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- 13.Bravou V, Klironomos G, Papadaki E, Taraviras S, Varakis J. ILK over-expression in human colon cancer progression correlates with activation of beta-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol. 2006;208:91–9. doi: 10.1002/path.1860. [DOI] [PubMed] [Google Scholar]
- 14.Wong RPC, Ng P, Dedhar S, Gang L. The role of integrin-linked kinase in melanoma cell migration, invasion, and tumor growth. Mol Cancer Ther. 2007;6:1692–700. doi: 10.1158/1535-7163.MCT-07-0134. [DOI] [PubMed] [Google Scholar]
- 15.Okamura M, Yamaji S, Nagashima Y, et al. Prognostic value of integrin beta1-ILK-pAkt signaling pathway in non-small cell lung cancer. Hum Pathol. 2007;38:1081–91. doi: 10.1016/j.humpath.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Gkretsi V, Bowen WC, Yang Y, Wu C, Michalopoulos GK. Integrin-linked kinase is involved in matrix-induced hepatocyte differentiation. Biochem Biophys Res Commun. 2007;16:638–43. doi: 10.1016/j.bbrc.2006.12.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone. A clinicopathologic study with emphasis on histologic grading. Cancer. 1977;40:818–913. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Bertoni F, Bacchini P, Hogendoorn PCW. Chondrosarcoma. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of soft tissue and bone. Lyon: IARC Press; 2002. pp. 247–51. [Google Scholar]
- 19.Papachristou DJ, Papachristou GI, Papaefthimiou OA, et al. The MAPK–AP-1/–Runx2 signalling axes are implicated in chondrosarcoma pathobiology either independently or via up-regulation of VEGF. Histopathology. 2005;47:565–74. doi: 10.1111/j.1365-2559.2005.02266.x. [DOI] [PubMed] [Google Scholar]
- 20.Persad S, Attwell S, Gray V, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 21.Persad S, Attwell S, Gray V, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 2000;97:3207–12. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Amico M, Hulit J, Amanatullah DF, et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649–57. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- 23.Grashoff G, Aszóoldi A, Sakai T, Hunziker EB, Fässler R. Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 2003;4:432–8. doi: 10.1038/sj.embor.embor801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongroo PS, Johnstone CN, Naruszewicz I, et al. Betα-parvin inhibits integrin-linked kinase signaling and is downregulated in breast cancer. Oncogene. 2004;23:8959–70. doi: 10.1038/sj.onc.1208112. [DOI] [PubMed] [Google Scholar]
- 25.Nikolopoulos SN, Turner CE. Molecular dissection of actopaxin-integrin-linked kinase-Paxillin interaction and their role in subcellular localization. J Biol Chem. 2002;277:1568–75. doi: 10.1074/jbc.M108612200. [DOI] [PubMed] [Google Scholar]
- 26.Shi X, Ma Y-Q, Tu Y, et al. The MIG-2/Integrin interaction strengthens cell–matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–66. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- 27.Papachristou DJ, Gkretsi V, Tu Y, et al. Increased cytoplasmic level of migfilin is associated with higher grades of human leiomyosarcoma. Histopathology. 2007;51:499–508. doi: 10.1111/j.1365-2559.2007.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlmann ER, Menendez LR, Fedenko AN, Learch T. Influence of cryosurgery on treatment outcome of low-grade chondrosarcoma. Clin Orthop Relat Res. 2006;451:201–7. doi: 10.1097/01.blo.0000229293.98850.5d. [DOI] [PubMed] [Google Scholar]
- 29.Leerapun T, Hugate RR, Inwards CY, Scully SP, Sim FH. Surgical management of conventional grade I chondrosarcoma of long bones. Clin Orthop Relat Res. 2007;463:166–72. doi: 10.1097/BLO.0b013e318146830f. [DOI] [PubMed] [Google Scholar]
- 30.Bjornsson J, McLeod RA, Unni KK, Ilstrup DM, Pritchard DJ. Primary chondrosarcoma of long bones and limb girdles. Cancer. 1998;83:2105–19. [PubMed] [Google Scholar]