Abstract
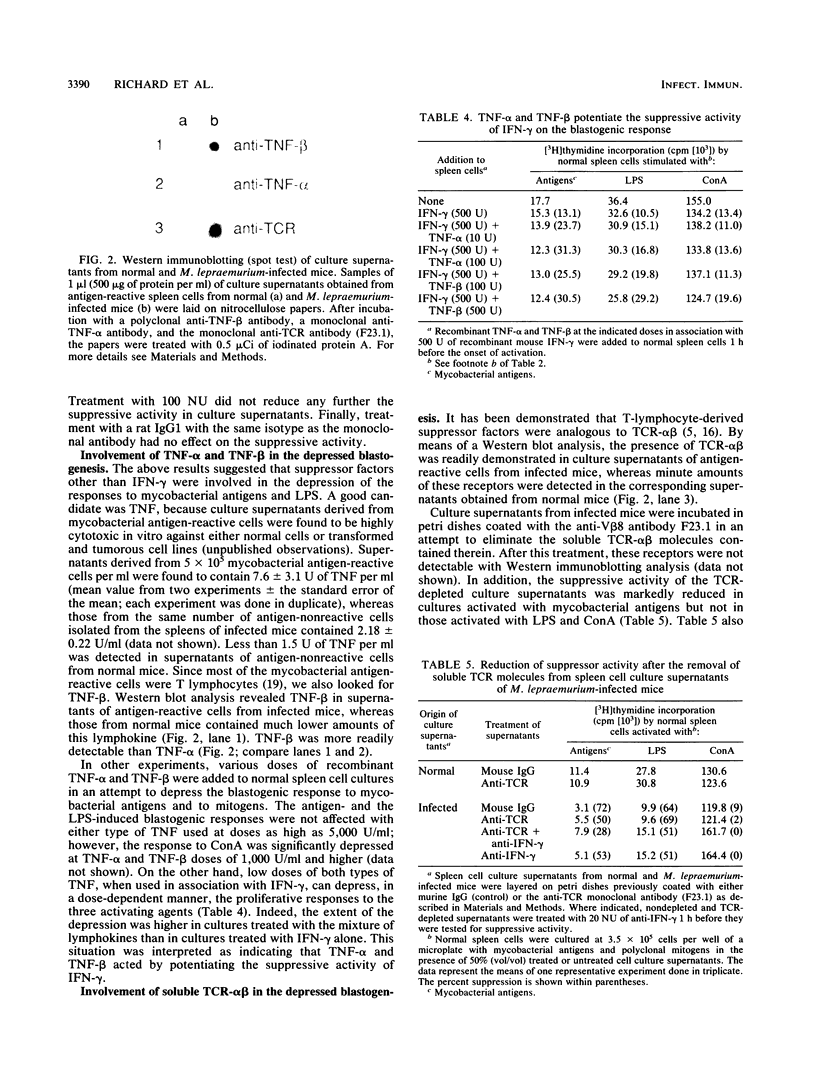
Spleen cells of Mycobacterium lepraemurium-infected mice were cultured on petri dishes coated with mycobacterial antigens, and antigen-reactive cells were isolated. Upon incubation in mitogen- or antigen-free culture medium, these cells released mediators capable of depressing the in vitro proliferative response of normal splenocytes to specific antigen and to concanavalin A and lipopolysaccharide. One of these mediators was identified with gamma interferon (IFN-gamma), mainly on the basis that treatment of supernatants with monoclonal anti-IFN-gamma antibodies markedly reduced the suppressive activity contained therein. Detectable levels of tumor necrosis factor alpha (TNF-alpha) and TNF-beta were present in spleen cell culture supernatants of infected mice. Moreover, low doses of recombinant TNF-alpha and TNF-beta were found to potentiate the suppressive activity of exogenous IFN-gamma. Soluble T-cell receptors beta were also detected in the culture supernatants. The elimination of these molecules with monoclonal anti-T-cell receptor beta (F23.1) antibodies immobilized on a plastic surface partially reversed the depression of the response to mycobacterial antigen but did not affect the response to mitogens. These results revealed the complex nature of suppressor mediators that are produced by mycobacterial antigen-reactive cells and that regulate the in vitro proliferative response.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Freeman G. L., Jr, Colley D. G. Production of the lymphokine soluble immune response suppressor (SIRS) during chronic experimental schistosomiasis mansoni. J Immunol. 1985 Oct;135(4):2768–2771. [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild R. L., Kubo R. T., Moorhead J. W. Soluble factors in tolerance and contact sensitivity to 2,4-dinitro-fluorobenzene in mice. IX. A monoclonal T cell suppressor molecule is structurally and serologically related to the alpha/beta T cell receptor. J Immunol. 1988 Nov 15;141(10):3342–3348. [PubMed] [Google Scholar]
- Friedman R. M., Vogel S. N. Interferons with special emphasis on the immune system. Adv Immunol. 1983;34:97–140. doi: 10.1016/s0065-2776(08)60378-8. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Weigle W. O. The regulatory effects of cytokines on the induction of a peripheral immunologic tolerance in mice. J Immunol. 1990 Sep 1;145(5):1318–1323. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Aggarwal B. B., Rinderknecht E., Assisi F., Chiu H. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083–1086. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Finkelman F. D., Sarma C., Ohara J., Serrate S. Recombinant interferon-gamma inhibits the B cell proliferative response stimulated by soluble but not by Sepharose-bound anti-immunoglobulin antibody. J Immunol. 1985 Oct;135(4):2513–2517. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Perrin P. J., Prystowsky M. B., Phillips S. M. The molecular basis of granuloma formation in schistosomiasis. II. Analogies of a T cell-derived suppressor effector factor to the T cell receptor. J Immunol. 1989 Feb 1;142(3):985–991. [PubMed] [Google Scholar]
- Powell M. B., Conta B. S., Horowitz M., Ruddle N. H. The differential inhibitory effect of lymphotoxin and immune interferon on normal and malignant lymphoid cells. Lymphokine Res. 1985 Winter;4(1):13–26. [PubMed] [Google Scholar]
- Richard L., Forget A., Turcotte R. Biological properties of factors secreted by antigen-reactive suppressor cells in mice infected with Mycobacterium lepraemurium. Infect Immun. 1990 Nov;58(11):3531–3536. doi: 10.1128/iai.58.11.3531-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L., Forget A., Turcotte R. Partial characterization of suppressor factors in spleen cell culture supernatants of Mycobacterium lepraemurium-infected mice. Adv Exp Med Biol. 1988;239:279–285. doi: 10.1007/978-1-4757-5421-6_28. [DOI] [PubMed] [Google Scholar]
- Siegel J. P. Effects of interferon-gamma on the activation of human T lymphocytes. Cell Immunol. 1988 Feb;111(2):461–472. doi: 10.1016/0008-8749(88)90109-8. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Sultzer B. M. Infection with Bacillus Calmette-Guérin activates murine thymus-independent (B) lymphocytes. J Immunol. 1978 Jan;120(1):254–261. [PubMed] [Google Scholar]
- Tomioka H., Saito H., Yamada Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J Gen Microbiol. 1990 May;136(5):965–973. doi: 10.1099/00221287-136-5-965. [DOI] [PubMed] [Google Scholar]
- Turcotte R., Lemieux S. Mechanisms of action of Mycobacterium bovis BCG-induced suppressor cells in mitogen-induced blastogenesis. Infect Immun. 1982 Apr;36(1):263–270. doi: 10.1128/iai.36.1.263-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Suppressor cells in experimental murine leprosy. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):358–363. [PubMed] [Google Scholar]
- Williamson B. D., Carswell E. A., Rubin B. Y., Prendergast J. S., Old L. J. Human tumor necrosis factor produced by human B-cell lines: synergistic cytotoxic interaction with human interferon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5397–5401. doi: 10.1073/pnas.80.17.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]