Abstract
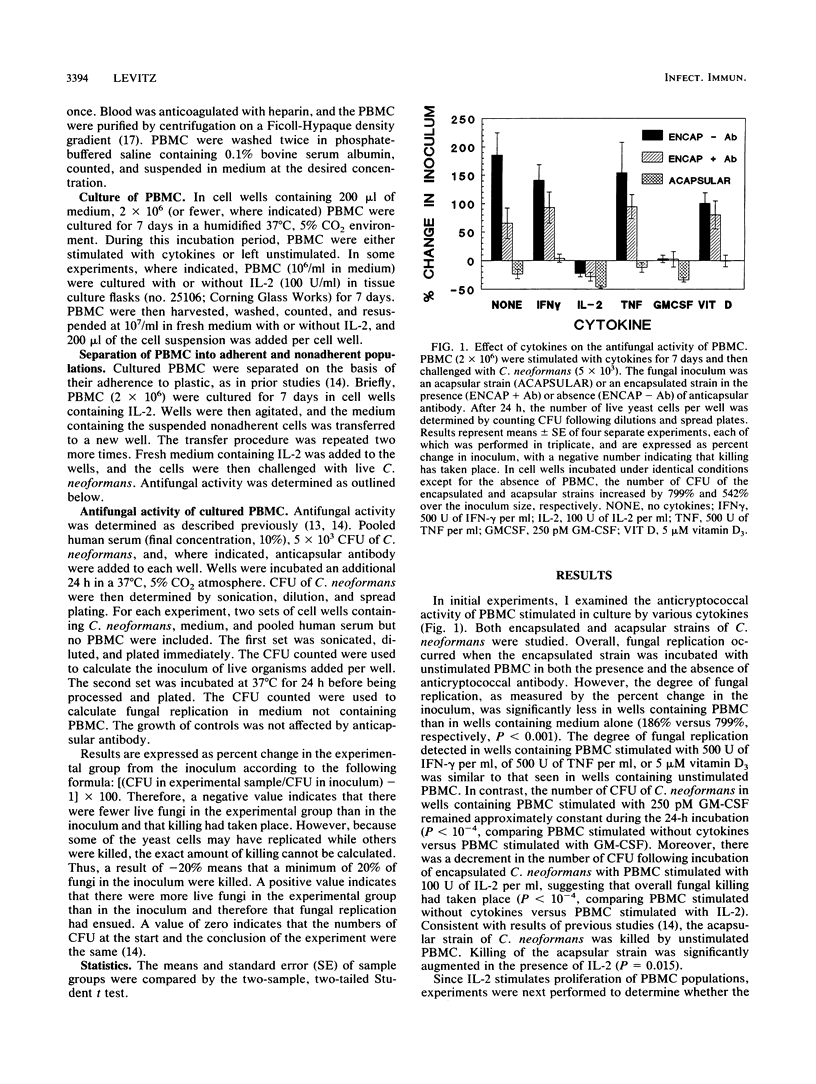
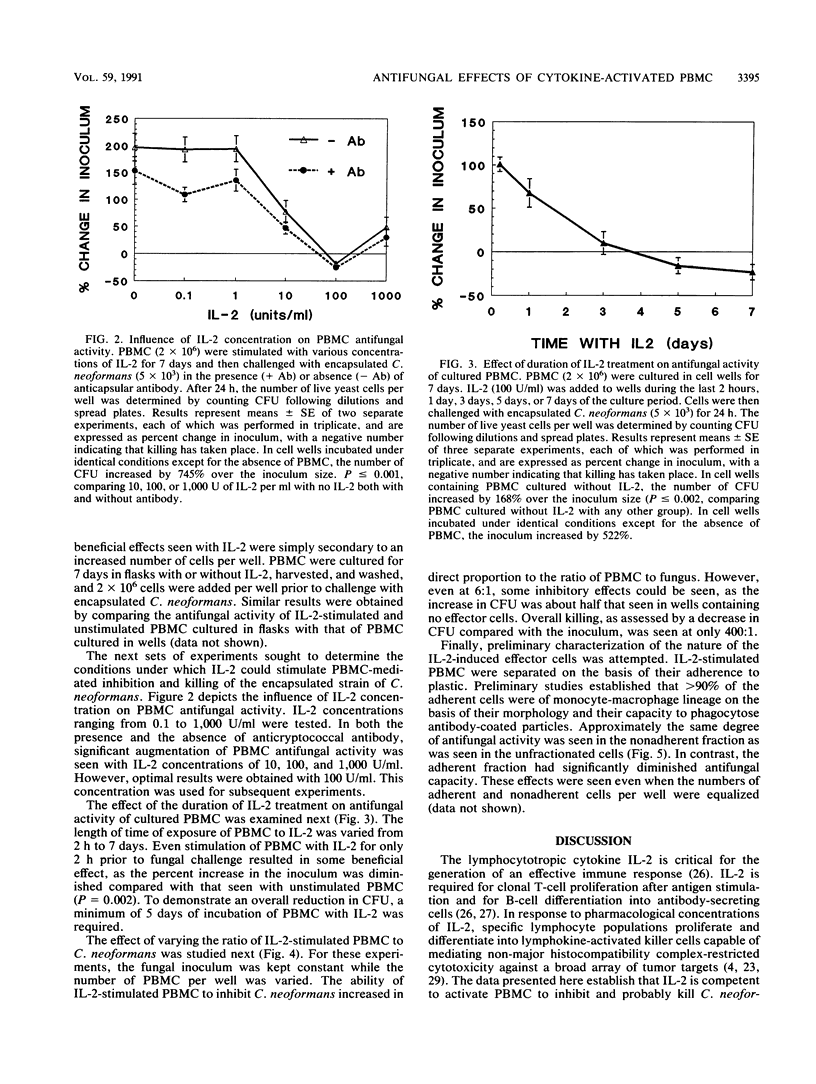
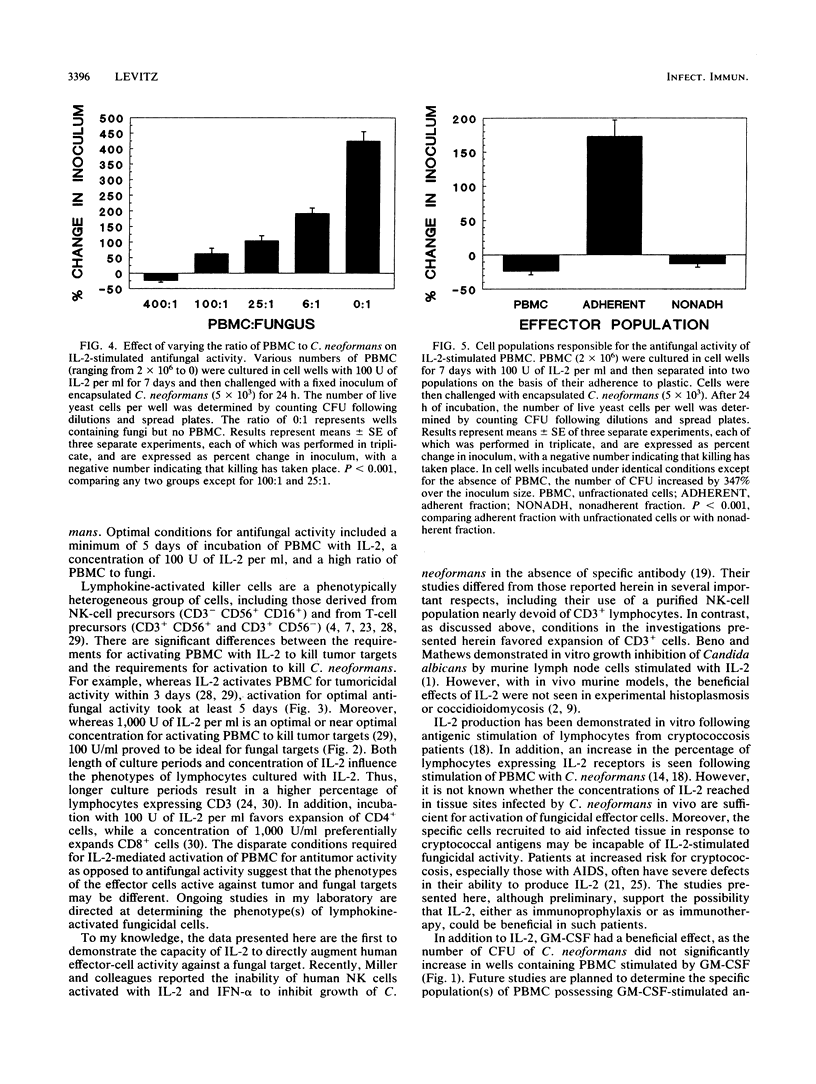
The abilities of selected cytokines to activate human peripheral blood mononuclear cells (PBMC) to inhibit and kill the opportunistic fungus Cryptococcus neoformans were studied. PBMC were cultured for 7 days in cell wells containing no cytokines, tumor necrosis factor (TNF), gamma interferon (IFN-gamma), 1,25-dihydroxycholecalciferol (vitamin D3), granulocyte-macrophage colony-stimulating factor (GM-CSF), or interleukin-2 (IL-2) and were then challenged for 24 h with a fixed number of CFU of C. neoformans. The number of CFU increased in wells containing no cytokines, TNF, IFN-gamma, or vitamin D3 and remained about the same in wells containing GM-CSF. In contrast, the number of CFU in wells containing IL-2-stimulated PBMC decreased, suggesting fungicidal activity. Optimal conditions for IL-2 stimulation included a minimum of 5 days of incubation of PBMC with IL-2, a concentration of 100 U of IL-2 per ml, and a high ratio of effectors to fungi. Separation of IL-2-stimulated PBMC based upon their adherence to plastic revealed that antifungal activity resided in the nonadherent fraction. These data demonstrate that IL-2 and GM-CSF are capable of stimulating PBMC-mediated antifungal activity and suggest that these cytokines may play physiological or pharmacological roles in host defenses against cryptococcosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beno D. W., Mathews H. L. Growth inhibition of Candida albicans by interleukin-2-induced lymph node cells. Cell Immunol. 1990 Jun;128(1):89–100. doi: 10.1016/0008-8749(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Taylor C. L., Harris J. E., Bullock W. E. Modulation of cellular immune responses in mice with disseminated histoplasmosis by recombinant interleukin-2. Infect Immun. 1986 Jul;53(1):6–12. doi: 10.1128/iai.53.1.6-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch P., Malkovsky M., Braakman E., Sturm E., Bolhuis R. L., Prieve A., Sosman J. A., Lam V. A., Sondel P. M. Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990 May 1;171(5):1567–1579. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Fromtling R. A., Shadomy H. J., Jacobson E. S. Decreased virulence in stable, acapsular mutants of cryptococcus neoformans. Mycopathologia. 1982 Jul 23;79(1):23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- Gerosa F., Tommasi M., Spiazzi A. L., Azzolina L. S., Carra G., Maffei A., Accolla R. S., Tridente G. Heterogeneity of lymphokine-activated killer (LAK) populations at the clonal level: both NK and CD3+, CD4-, CD8- clones efficiently mediate tumor cell killing. Clin Immunol Immunopathol. 1988 Oct;49(1):91–100. doi: 10.1016/0090-1229(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Hoeprich P. D., Merry J. M. Effect of recombinant human interleukin 2 in experimental murine coccidioidomycosis. Diagn Microbiol Infect Dis. 1988 Feb;9(2):115–118. doi: 10.1016/0732-8893(88)90104-6. [DOI] [PubMed] [Google Scholar]
- Jacobson E. S., Ayers D. J., Harrell A. C., Nicholas C. C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Differential stimulation of murine resident peritoneal cells by selectively opsonized encapsulated and acapsular Cryptococcus neoformans. Infect Immun. 1988 Oct;56(10):2544–2551. doi: 10.1128/iai.56.10.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., DiBenedetto D. J. Paradoxical role of capsule in murine bronchoalveolar macrophage-mediated killing of Cryptococcus neoformans. J Immunol. 1989 Jan 15;142(2):659–665. [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P. Growth inhibition of Cryptococcus neoformans by cultured human monocytes: role of the capsule, opsonins, the culture surface, and cytokines. Infect Immun. 1990 May;58(5):1201–1209. doi: 10.1128/iai.58.5.1201-1209.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P., Maziarz R. T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991 May;163(5):1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Tabuni A. Binding of Cryptococcus neoformans by human cultured macrophages. Requirements for multiple complement receptors and actin. J Clin Invest. 1991 Feb;87(2):528–535. doi: 10.1172/JCI115027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. P., Puck J. In vitro human lymphocyte responses to Cryptococcus neoformans. Evidence for primary and secondary responses in normals and infected subjects. J Immunol. 1984 Jul;133(1):166–172. [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Murray H. W., Welte K., Jacobs J. L., Rubin B. Y., Mertelsmann R., Roberts R. B. Production of and in vitro response to interleukin 2 in the acquired immunodeficiency syndrome. J Clin Invest. 1985 Nov;76(5):1959–1964. doi: 10.1172/JCI112194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R. Cryptococcosis. Infect Dis Clin North Am. 1989 Mar;3(1):77–102. [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel E., Gerrard J. M., Greenberg A. H. Long-term cultures of human peripheral blood lymphocytes with recombinant human interleukin-2 generate a population of virtually pure CD3+ CD16- CD56- large granular lymphocyte LAK cells. Clin Exp Immunol. 1990 Nov;82(2):416–421. doi: 10.1111/j.1365-2249.1990.tb05463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann M., Pinching A. J., Rosen F. S., Fahey J. L., Khaitov R. M., Klatzmann D., Koenig S., Luo N., Ngu J., Riethmüller G. Immunology of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. An update. Ann Intern Med. 1987 Aug;107(2):234–242. doi: 10.7326/0003-4819-107-2-234. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Tigges M. A., Casey L. S., Koshland M. E. Mechanism of interleukin-2 signaling: mediation of different outcomes by a single receptor and transduction pathway. Science. 1989 Feb 10;243(4892):781–786. doi: 10.1126/science.2492678. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Itoh K., Balch C. M. Human lymphokine-activated killer (LAK) cells: identification of two types of effector cells. J Immunol. 1987 Feb 15;138(4):1068–1073. [PubMed] [Google Scholar]
- Winkelstein A., Weaver L. D., Salva N., Machen L. L. Interleukin-2-induced lymphoproliferative responses. Cancer Immunol Immunother. 1990;32(2):110–116. doi: 10.1007/BF01754207. [DOI] [PMC free article] [PubMed] [Google Scholar]


