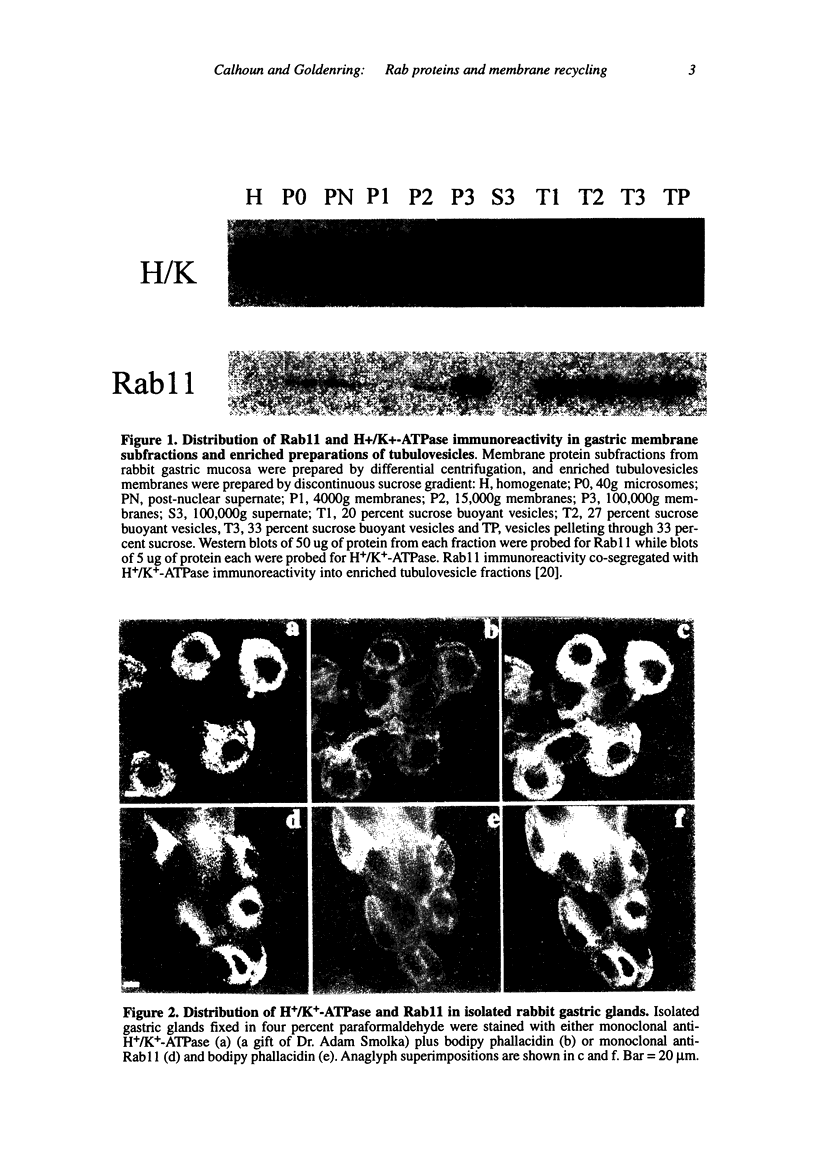
Abstract
The gastric parietal cell secretes large quantities of HCl into the lumen of the gastric gland in response to secretagogues such as histamine. In the membrane recycling hypothesis, this secretory activity requires the trafficking of the gastric H+/K(+)-ATPase to the cell surface from intracellular tubulovesicles. The Rab subclass of small GTP-binding proteins is thought to confer specificity to vesicle transport throughout the secretory pathway, and previous investigations established that Rab11 is highly expressed in gastric parietal cells. Recent discoveries in intra-Golgi transport and neuronal synaptic vesicle fusion have fortuitously converged on an evolutionarily conserved protein complex involved in vesicle docking and fusion. Recent results indicate that Rab11 is involved in the apical targeting of vesicles in parietal cells and other epithelial cells throughout the gastrointestinal tract. In support of the membrane recycling hypothesis, Rab co-segregates with H+/K(+)-ATPase in parietal cells. The presence of Rab11 on tubulovesicles supports a role for this Rab protein in recycling vesicle trafficking.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basson M. D., Goldenring J. R., Tang L. H., Lewis J. J., Padfield P., Jamieson J. D., Modlin I. M. Redistribution of 23 kDa tubulovesicle-associated GTP-binding proteins during parietal cell stimulation. Biochem J. 1991 Oct 1;279(Pt 1):43–48. doi: 10.1042/bj2790043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Calakos N., Scheller R. H. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992 Jul 10;257(5067):255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., García-Arrarás J. E., Elferink L. A., Peterson K., Fleming A. M., Hazuka C. D., Scheller R. H. The syntaxin family of vesicular transport receptors. Cell. 1993 Sep 10;74(5):863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. The molecular machinery for secretion is conserved from yeast to neurons. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2559–2563. doi: 10.1073/pnas.90.7.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Black J. A., Forte T. M., Forte J. G. Structure of oxyntic cell membranes during conditions of rest and secretion of HCl as revealed by freeze-fracture. Anat Rec. 1980 Feb;196(2):163–172. doi: 10.1002/ar.1091960206. [DOI] [PubMed] [Google Scholar]
- Blasi J., Binz T., Yamasaki S., Link E., Niemann H., Jahn R. Inhibition of neurotransmitter release by clostridial neurotoxins correlates with specific proteolysis of synaptosomal proteins. J Physiol Paris. 1994;88(4):235–241. doi: 10.1016/0928-4257(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman E. R., Link E., Binz T., Yamasaki S., De Camilli P., Südhof T. C., Niemann H., Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993 Sep 9;365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Block M. R., Glick B. S., Wilcox C. A., Wieland F. T., Rothman J. E. Purification of an N-ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Griff I. C., Rothman J. E. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990 May 18;61(4):709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- Darchen F., Zahraoui A., Hammel F., Monteils M. P., Tavitian A., Scherman D. Association of the GTP-binding protein Rab3A with bovine adrenal chromaffin granules. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5692–5696. doi: 10.1073/pnas.87.15.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBona D. R., Ito S., Berglindh T., Sachs G. Cellular site of gastric acid secretion. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6689–6693. doi: 10.1073/pnas.76.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Jahn R. Vesicle fusion from yeast to man. Nature. 1994 Jul 21;370(6486):191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Machen T. E., Forte J. G. Ultrastructural changes in oxyntic cells associated with secretory function: a membrane-recycling hypothesis. Gastroenterology. 1977 Oct;73(4 Pt 2):941–955. [PubMed] [Google Scholar]
- Gaisano H. Y., Sheu L., Foskett J. K., Trimble W. S. Tetanus toxin light chain cleaves a vesicle-associated membrane protein (VAMP) isoform 2 in rat pancreatic zymogen granules and inhibits enzyme secretion. J Biol Chem. 1994 Jun 24;269(25):17062–17066. [PubMed] [Google Scholar]
- Goldenring J. R., Shen K. R., Vaughan H. D., Modlin I. M. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem. 1993 Sep 5;268(25):18419–18422. [PubMed] [Google Scholar]
- Goldenring J. R., Smith J., Vaughan H. D., Cameron P., Hawkins W., Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996 Mar;270(3 Pt 1):G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Soroka C. J., Shen K. R., Tang L. H., Rodriguez W., Vaughan H. D., Stoch S. A., Modlin I. M. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am J Physiol. 1994 Aug;267(2 Pt 1):G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- Gorelick F. S., Cohn J. A., Freedman S. D., Delahunt N. G., Gershoni J. M., Jamieson J. D. Calmodulin-stimulated protein kinase activity from rat pancreas. J Cell Biol. 1983 Oct;97(4):1294–1298. doi: 10.1083/jcb.97.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel D. K., Urushidani T., Usinger W. R., Smolka A., Forte J. G. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol. 1989 Jun;256(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1989.256.6.G1082. [DOI] [PubMed] [Google Scholar]
- Hayashi T., McMahon H., Yamasaki S., Binz T., Hata Y., Südhof T. C., Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994 Nov 1;13(21):5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst B. H., Forte J. G. Redistribution and characterization of (H+ + K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J. 1985 Nov 1;231(3):641–649. doi: 10.1042/bj2310641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena B. P., Gumkowski F. D., Konieczko E. M., von Mollard G. F., Jahn R., Jamieson J. D. Redistribution of a rab3-like GTP-binding protein from secretory granules to the Golgi complex in pancreatic acinar cells during regulated exocytosis. J Cell Biol. 1994 Jan;124(1-2):43–53. doi: 10.1083/jcb.124.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam S. M., Forte J. G. Inhibiting gastric H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am J Physiol. 1994 Apr;266(4 Pt 1):G745–G758. doi: 10.1152/ajpgi.1994.266.4.G745. [DOI] [PubMed] [Google Scholar]
- Link E., Edelmann L., Chou J. H., Binz T., Yamasaki S., Eisel U., Baumert M., Südhof T. C., Niemann H., Jahn R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Orci L., Glick B. S., Block M. R., Rothman J. E. Role of an N-ethylmaleimide-sensitive transport component in promoting fusion of transport vesicles with cisternae of the Golgi stack. Cell. 1988 Jul 15;54(2):221–227. doi: 10.1016/0092-8674(88)90554-5. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Südhof T. C. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity alpha-SNAP binding site. J Biol Chem. 1995 Feb 3;270(5):2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Balch W. E. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J. M., Humphris D. C., Barrett S. P., Toh B. H., van Driel I. R., Gleeson P. A. Fast freeze-fixation/freeze-substitution reveals the secretory membranes of the gastric parietal cell as a network of helically coiled tubule. A new model for parietal cell transformation. J Cell Sci. 1995 Mar;108(Pt 3):1127–1141. doi: 10.1242/jcs.108.3.1127. [DOI] [PubMed] [Google Scholar]
- Roth D., Burgoyne R. D. SNAP-25 is present in a SNARE complex in adrenal chromaffin cells. FEBS Lett. 1994 Sep 5;351(2):207–210. doi: 10.1016/0014-5793(94)00833-7. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Shone C. C., Bennett M. K., Scheller R. H., Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995 May 5;270(18):10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Soroka C. J., Chew C. S., Hanzel D. K., Smolka A., Modlin I. M., Goldenring J. R. Characterization of membrane and cytoskeletal compartments in cultured parietal cells: immunofluorescence and confocal microscopic examination. Eur J Cell Biol. 1993 Feb;60(1):76–87. [PubMed] [Google Scholar]
- Söllner T., Bennett M. K., Whiteheart S. W., Scheller R. H., Rothman J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993 Nov 5;75(3):409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Stoch S. A., Modlin I. M., Goldenring J. R. Identification of rab2 as a tubulovesicle-membrane-associated protein in rabbit gastric parietal cells. Biochem J. 1992 Aug 1;285(Pt 3):715–719. doi: 10.1042/bj2850715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Cowan D. M., Scheller R. H. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushidani T., Forte J. G. Stimulation-associated redistribution of H+-K+-ATPase activity in isolated gastric glands. Am J Physiol. 1987 Apr;252(4 Pt 1):G458–G465. doi: 10.1152/ajpgi.1987.252.4.G458. [DOI] [PubMed] [Google Scholar]
- Weidman P. J., Melançon P., Block M. R., Rothman J. E. Binding of an N-ethylmaleimide-sensitive fusion protein to Golgi membranes requires both a soluble protein(s) and an integral membrane receptor. J Cell Biol. 1989 May;108(5):1589–1596. doi: 10.1083/jcb.108.5.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Whiteheart S. W., Wiedmann M., Brunner M., Rothman J. E. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992 May;117(3):531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Chaponnier C., Gabbiani G., Forte J. G. Polarized distribution of actin isoforms in gastric parietal cells. Mol Biol Cell. 1995 May;6(5):541–557. doi: 10.1091/mbc.6.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]