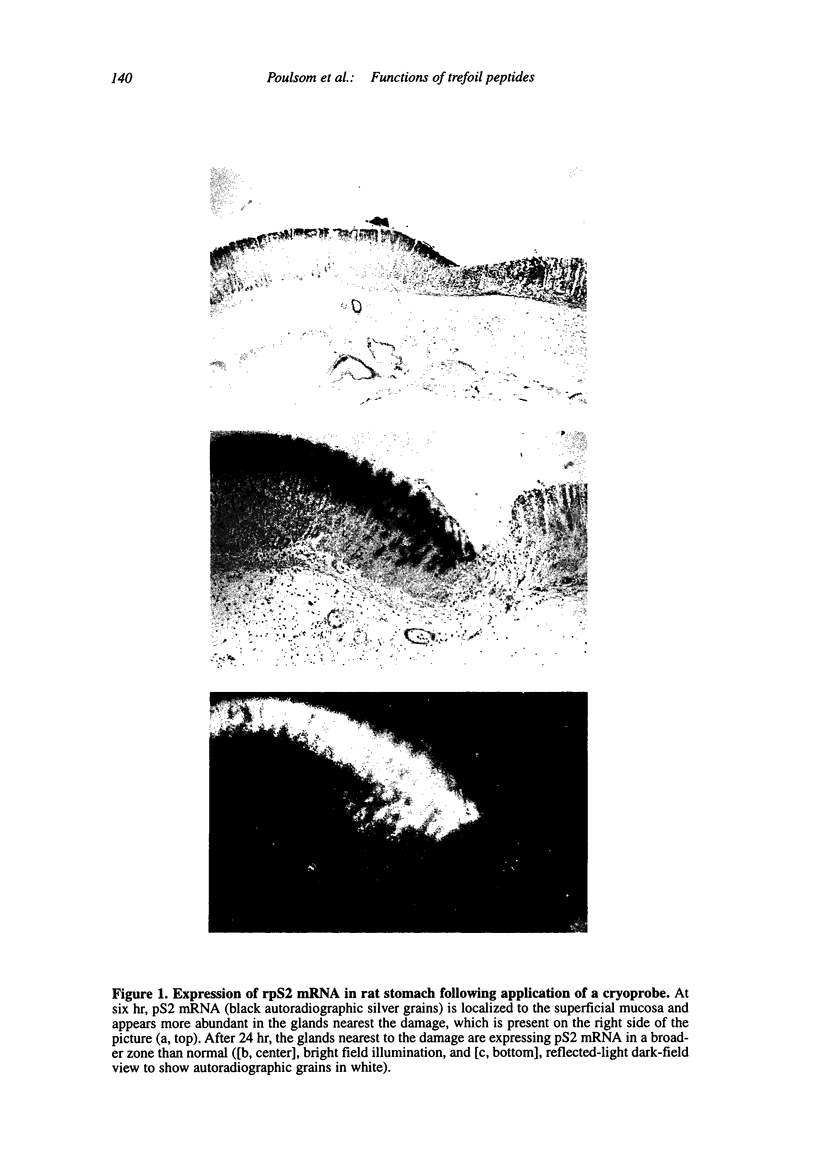
Abstract
Healing of mucosal damage takes place in two phases: restitution of mucosal integrity and remodeling towards recreating the original glandular arrangements. These processes can be observed in several experimental rodent models: e.g., cryoprobe or NSAID-generated ulcers in the gastric or duodenal mucosa and following surgical resection of the small or large bowel. In some studies, it has been possible to detect changes in the expression of peptides, either in the reparative epithelium or adjacent to the damage, that may contribute to the healing processes. Trefoil peptides are expressed constitutively by epithelial cells in specific regions of the gastrointestinal tract, in association with mucins. Several studies have shown that trefoil peptide expression is enhanced at sites of damage in man and rat, and experimental evidence supports their active participation in the healing process. Recombinant trefoil peptides are able to enhance the rate of epithelial cell migration in vitro and are able to protect against indomethacin-induced damage in vivo, yet they do not depend upon TGF-beta for enhancing cell migration and do not appear to affect acid secretion. The mode of action of trefoil peptides appears to be receptor-mediated but is not simple. There is good evidence that there are interactions between members of the trefoil family and the EGF family that are beneficial for mucosal defense and repair. This raises the possibility that combining trefoil peptides with other growth factors or small molecules may be advantageous for treatment of ulceration.
Full text
PDF


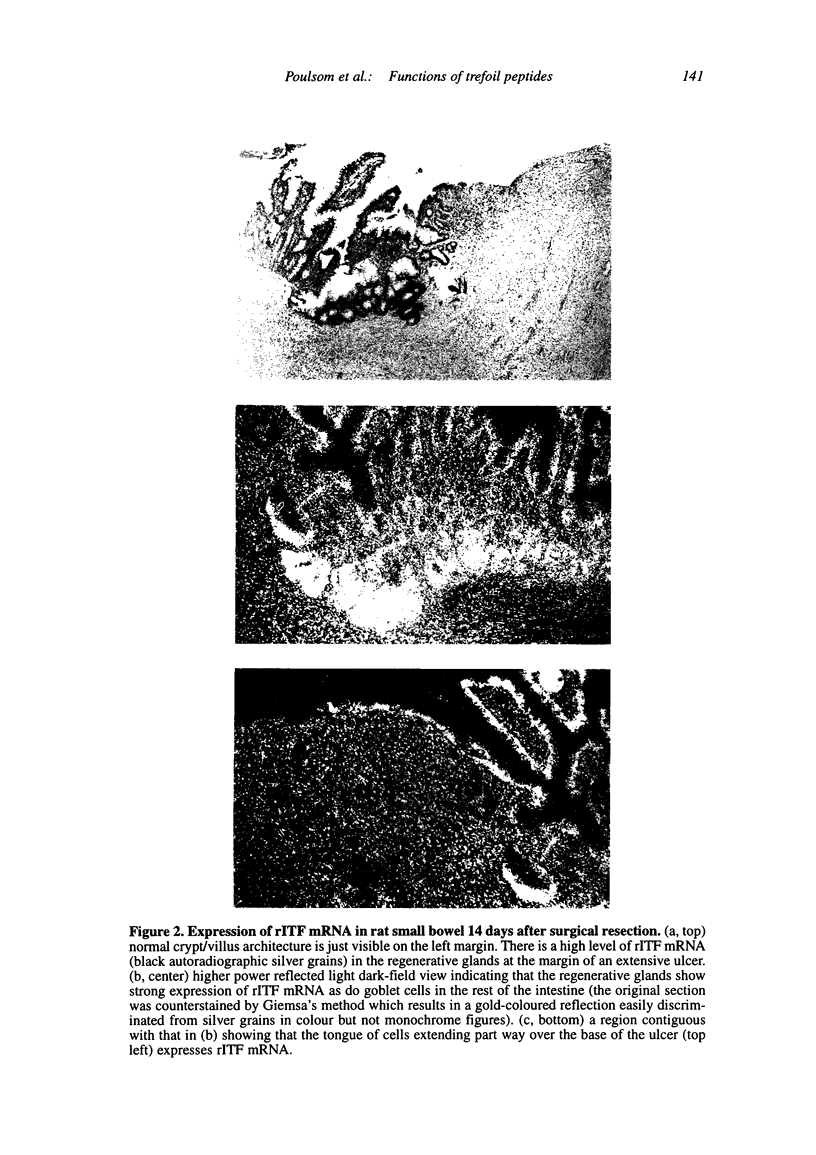





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H., Stein U., Welter C., Remberger K. Differential expression of the pS2 protein in the human prostate and prostate cancer: association with premalignant changes and neuroendocrine differentiation. Hum Pathol. 1995 Aug;26(8):824–828. doi: 10.1016/0046-8177(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Carr M. D., Bauer C. J., Gradwell M. J., Feeney J. Solution structure of a trefoil-motif-containing cell growth factor, porcine spasmolytic protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2206–2210. doi: 10.1073/pnas.91.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles V., Garcia M., Rochefort H. Regulation of cathepsin-D and pS2 gene expression by growth factors in MCF7 human breast cancer cells. Mol Endocrinol. 1989 Mar;3(3):552–558. doi: 10.1210/mend-3-3-552. [DOI] [PubMed] [Google Scholar]
- Chadwick M. P., May F. E., Westley B. R. Production and comparison of mature single-domain 'trefoil' peptides pNR-2/pS2 Cys58 and pNR-2/pS2 Ser58. Biochem J. 1995 Jun 15;308(Pt 3):1001–1007. doi: 10.1042/bj3081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinery R., Bates P. A., De A., Freemont P. S. Characterisation of the single copy trefoil peptides intestinal trefoil factor and pS2 and their ability to form covalent dimers. FEBS Lett. 1995 Jan 2;357(1):50–54. doi: 10.1016/0014-5793(94)01297-e. [DOI] [PubMed] [Google Scholar]
- Chinery R., Cox H. M. Immunoprecipitation and characterization of a binding protein specific for the peptide, intestinal trefoil factor. Peptides. 1995;16(4):749–755. doi: 10.1016/0196-9781(95)00045-l. [DOI] [PubMed] [Google Scholar]
- Chinery R., Cox H. M. Modulation of epidermal growth factor effects on epithelial ion transport by intestinal trefoil factor. Br J Pharmacol. 1995 May;115(1):77–80. doi: 10.1111/j.1476-5381.1995.tb16322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinery R., Playford R. J. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995 Apr;88(4):401–403. doi: 10.1042/cs0880401. [DOI] [PubMed] [Google Scholar]
- Chinery R., Poulsom R., Elia G., Hanby A. M., Wright N. A. Expression and purification of a trefoil peptide motif in a beta-galactosidase fusion protein and its use to search for trefoil-binding sites. Eur J Biochem. 1993 Mar 1;212(2):557–563. doi: 10.1111/j.1432-1033.1993.tb17693.x. [DOI] [PubMed] [Google Scholar]
- Chinery R., Poulsom R., Rogers L. A., Jeffery R. E., Longcroft J. M., Hanby A. M., Wright N. A. Localization of intestinal trefoil-factor mRNA in rat stomach and intestine by hybridization in situ. Biochem J. 1992 Jul 1;285(Pt 1):5–8. doi: 10.1042/bj2850005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A., Brown D. G., Gorman M. A., Carr M., Sanderson M. R., Freemont P. S. Crystal structure of a disulfide-linked "trefoil" motif found in a large family of putative growth factors. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1084–1088. doi: 10.1073/pnas.91.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A. U., Podolsky D. K. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993 Nov;105(5):1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Chinery R., Hanby A. M., Poulsom R., Wright N. A. The production and characterization of a new monoclonal antibody to the trefoil peptide human spasmolytic polypeptide. Histochem J. 1994 Aug;26(8):644–647. doi: 10.1007/BF00158289. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Jørgensen K. H., Thim L. Receptor binding of pancreatic spasmolytic polypeptide (PSP) in rat intestinal mucosal cell membranes inhibits the adenylate cyclase activity. Regul Pept. 1986 Dec 30;16(3-4):291–297. doi: 10.1016/0167-0115(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K. Receptor binding of pancreatic spasmolytic polypeptide in intestinal mucosal cells and membranes. Regul Pept. 1988 Jan;20(1):45–52. doi: 10.1016/0167-0115(88)90056-0. [DOI] [PubMed] [Google Scholar]
- Gajhede M., Petersen T. N., Henriksen A., Petersen J. F., Dauter Z., Wilson K. S., Thim L. Pancreatic spasmolytic polypeptide: first three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure. 1993 Dec 15;1(4):253–262. doi: 10.1016/0969-2126(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Hauser F., Gertzen E. M., Hoffmann W. Expression of spasmolysin (FIM-A.1): an integumentary mucin from Xenopus laevis. Exp Cell Res. 1990 Aug;189(2):157–162. doi: 10.1016/0014-4827(90)90230-8. [DOI] [PubMed] [Google Scholar]
- Hauser F., Poulsom R., Chinery R., Rogers L. A., Hanby A. M., Wright N. A., Hoffmann W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Hauser F. The P-domain or trefoil motif: a role in renewal and pathology of mucous epithelia? Trends Biochem Sci. 1993 Jul;18(7):239–243. doi: 10.1016/0968-0004(93)90170-r. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Thim L., Jørgensen K. H., Brattain M. G. Growth stimulatory effect of pancreatic spasmolytic polypeptide on cultured colon and breast tumor cells. FEBS Lett. 1989 Apr 24;247(2):303–306. doi: 10.1016/0014-5793(89)81357-2. [DOI] [PubMed] [Google Scholar]
- Hull M. A., Cullen D. J., Hudson N., Hawkey C. J. Basic fibroblast growth factor treatment for non-steroidal anti-inflammatory drug associated gastric ulceration. Gut. 1995 Nov;37(5):610–612. doi: 10.1136/gut.37.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A., Sandborg R. R., Horwitz A. F. Adhesion in cell migration. Curr Opin Cell Biol. 1995 Oct;7(5):697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Jeffrey G. P., Oates P. S., Wang T. C., Babyatsky M. W., Brand S. J. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994 Feb;106(2):336–345. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. D., Diamant B., Jørgensen K. H., Thim L. Pancreatic spasmolytic polypeptide (PSP): III. Pharmacology of a new porcine pancreatic polypeptide with spasmolytic and gastric acid secretion inhibitory effects. Regul Pept. 1982 Mar;3(3-4):231–243. doi: 10.1016/0167-0115(82)90128-8. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. H., Thim L., Jacobsen H. E. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982 Mar;3(3-4):207–219. doi: 10.1016/0167-0115(82)90126-4. [DOI] [PubMed] [Google Scholar]
- Kida N., Yoshimura T., Mori K., Hayashi K. Hormonal regulation of synthesis and secretion of pS2 protein relevant to growth of human breast cancer cells (MCF-7). Cancer Res. 1989 Jul 1;49(13):3494–3498. [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Morris G. P., Cohen M. M. Rapid repair of the surface epithelium in human gastric mucosa after acute superficial injury. J Clin Gastroenterol. 1993;17 (Suppl 1):S125–S135. doi: 10.1097/00004836-199312001-00023. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Kédinger M., Chenard M. P., Tomasetto C., Chambon P., Rio M. C. The mouse one P-domain (pS2) and two P-domain (mSP) genes exhibit distinct patterns of expression. J Cell Biol. 1993 Jul;122(1):191–198. doi: 10.1083/jcb.122.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita S., Hirota M., Yamamoto T., Shiroyama C., Furukawa Y., Hayashi K. Effect of basic fibroblast growth factor on synthesis/secretion of pS2 protein by human breast cancer cells (MCF-7). Eur J Biochem. 1994 Nov 1;225(3):1041–1046. doi: 10.1111/j.1432-1033.1994.1041b.x. [DOI] [PubMed] [Google Scholar]
- Miyashita S., Nomoto H., Konishi H., Hayashi K. Estimation of pS2 protein level in human body fluids by a sensitive two-site enzyme immunoassay. Clin Chim Acta. 1994 Aug;228(2):71–81. doi: 10.1016/0009-8981(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto W. R., Rao J., Cox H. M., Kotzian E., Lee C. Y., Goodlad R. A., Lane A., Gorman M., Freemont P. A., Hansen H. F. Effects of pancreatic spasmolytic Polypeptide (PSP) on epithelial cell function. Eur J Biochem. 1996 Jan 15;235(1-2):64–72. doi: 10.1111/j.1432-1033.1996.00064.x. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Poulsom R., Chinery R., Sarraf C., Lalani E. N., Stamp G., Elia G., Wright N. Trefoil peptide expression in intestinal adaptation and renewal. Scand J Gastroenterol Suppl. 1992;192:17–28. doi: 10.3109/00365529209095975. [DOI] [PubMed] [Google Scholar]
- Poulsom R., Wright N. A. Trefoil peptides: a newly recognized family of epithelial mucin-associated molecules. Am J Physiol. 1993 Aug;265(2 Pt 1):G205–G213. doi: 10.1152/ajpgi.1993.265.2.G205. [DOI] [PubMed] [Google Scholar]
- Prud'homme J. F., Jolivet A., Pichon M. F., Savouret J. F., Milgrom E. Monoclonal antibodies against native ant denatured forms of estrogen-induced breast cancer protein (BCEI/pS2) obtained by expression in Escherichia coli. Cancer Res. 1990 Apr 15;50(8):2390–2396. [PubMed] [Google Scholar]
- Rasmussen T. N., Raaberg L., Poulsen S. S., Thim L., Holst J. J. Immunohistochemical localization of pancreatic spasmolytic polypeptide (PSP) in the pig. Histochemistry. 1992 Sep;98(2):113–119. doi: 10.1007/BF00717002. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Schmassmann A., Tarnawski A., Peskar B. M., Varga L., Flogerzi B., Halter F. Influence of acid and angiogenesis on kinetics of gastric ulcer healing in rats: interaction with indomethacin. Am J Physiol. 1995 Feb;268(2 Pt 1):G276–G285. doi: 10.1152/ajpgi.1995.268.2.G276. [DOI] [PubMed] [Google Scholar]
- Suemori S., Lynch-Devaney K., Podolsky D. K. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L. A new family of growth factor-like peptides. 'Trefoil' disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989 Jun 19;250(1):85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- Thim L., Norris K., Norris F., Nielsen P. F., Bjørn S. E., Christensen M., Petersen J. Purification and characterization of the trefoil peptide human spasmolytic polypeptide (hSP) produced in yeast. FEBS Lett. 1993 Mar 8;318(3):345–352. doi: 10.1016/0014-5793(93)80543-4. [DOI] [PubMed] [Google Scholar]
- Thim L., Wöldike H. F., Nielsen P. F., Christensen M., Lynch-Devaney K., Podolsky D. K. Characterization of human and rat intestinal trefoil factor produced in yeast. Biochemistry. 1995 Apr 11;34(14):4757–4764. doi: 10.1021/bi00014a033. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetto C., Wolf C., Rio M. C., Mehtali M., LeMeur M., Gerlinger P., Chambon P., Lathe R. Breast cancer protein PS2 synthesis in mammary gland of transgenic mice and secretion into milk. Mol Endocrinol. 1989 Oct;3(10):1579–1584. doi: 10.1210/mend-3-10-1579. [DOI] [PubMed] [Google Scholar]
- Tomita M., Itoh H., Ishikawa N., Higa A., Ide H., Murakumo Y., Maruyama H., Koga Y., Nawa Y. Molecular cloning of mouse intestinal trefoil factor and its expression during goblet cell changes. Biochem J. 1995 Oct 1;311(Pt 1):293–297. doi: 10.1042/bj3110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]