Abstract
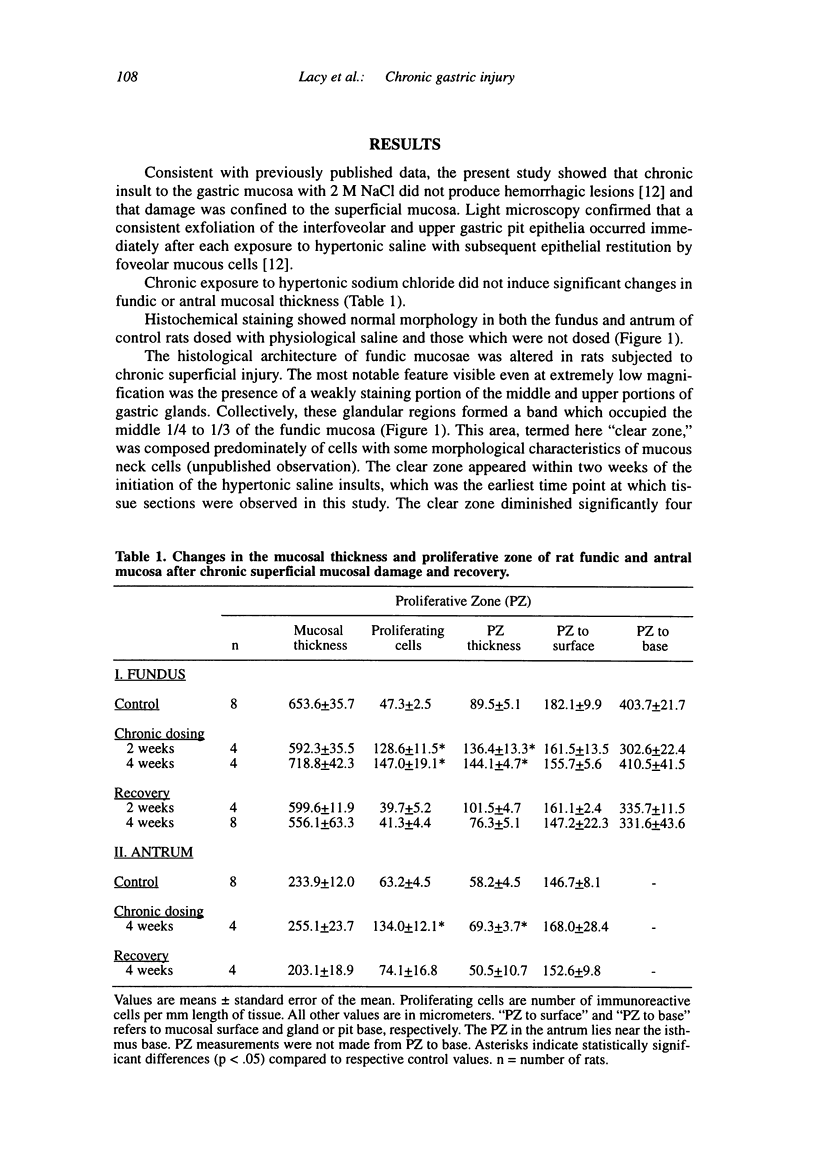
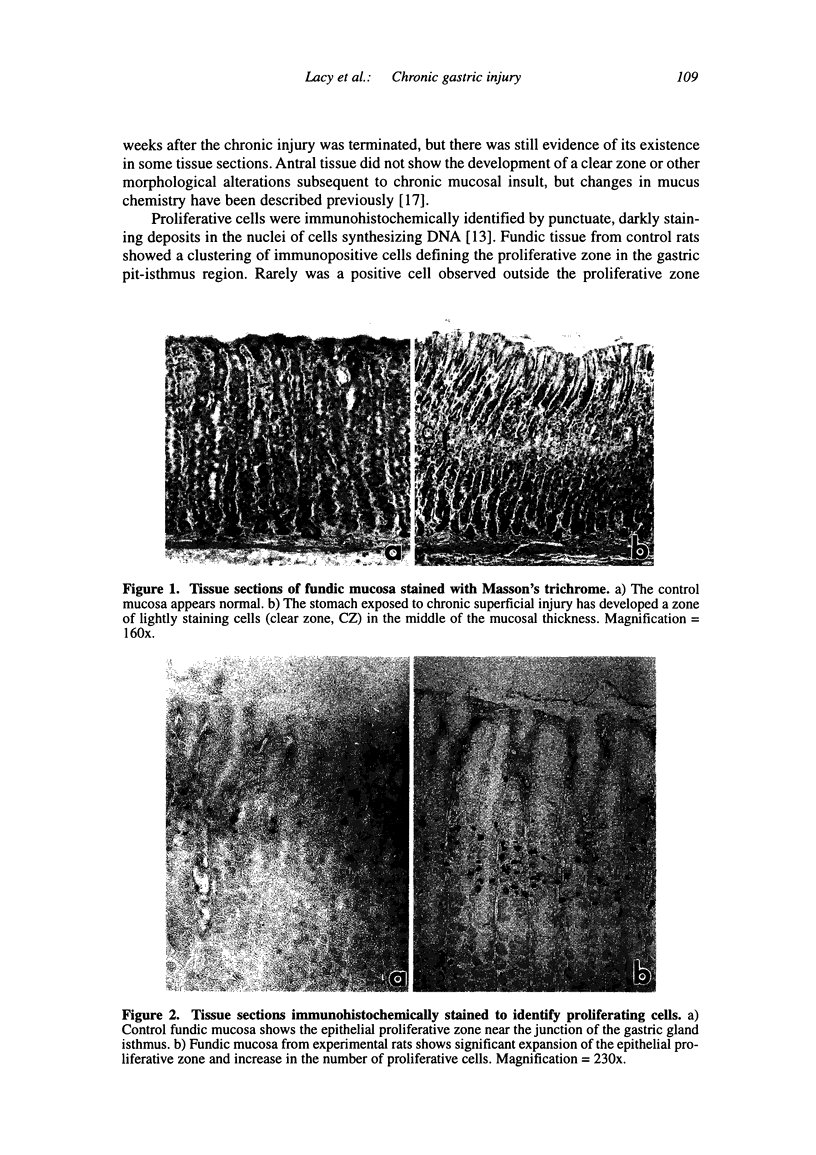
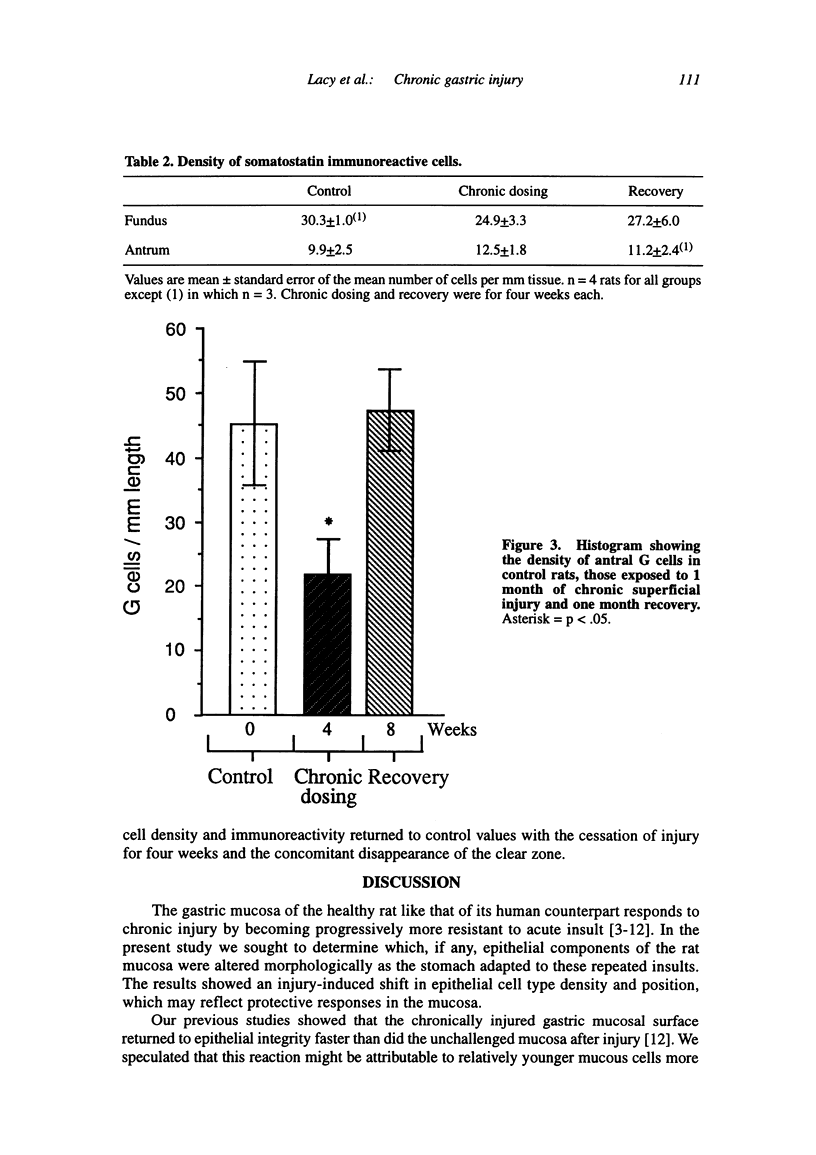
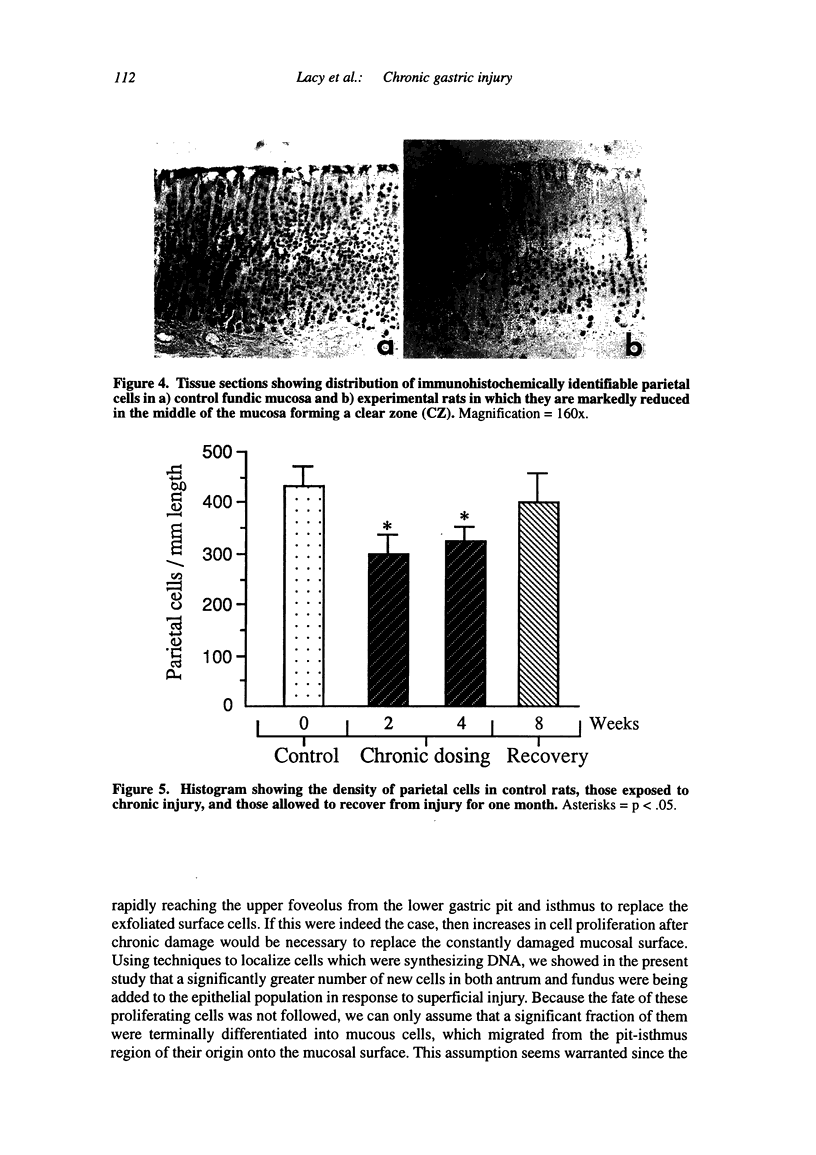
Chronic injury to the healthy gastric mucosa with noxious agents such as aspirin or alcohol induces a progressive strengthening of the stomach wall against these insults. The present study examined the histologic response of the rat gastric mucosa to chronic destruction of the superficial mucosa for one month with hypertonic saline. The number, position and morphology of proliferating, parietal, G and D cells were followed during mucosal injury and one month of recovery. The results showed that chronic injury reduced parietal cell numbers by about 30 percent, particularly in the middle of the mucosal thickness where a clear zone was formed by hypertrophy of mucous neck-like cells. G cells were also reduced by about 50 percent, but there were no changes in D cells. Chronic injury induced a marked increase in the number of antral (+112 percent) and fundic (+250 percent) proliferating cells. CONCLUSION: The rat gastric mucosa responds to chronic superficial injury by down-regulation of acid secretory cells and gastrin secreting cells and an up-regulation of proliferating cells. The appearance of a prominent layer of mucous neck-like cells may indicate a new secretory function for these cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aponte G., Gross D., Yamada T. Capillary orientation of rat pancreatic D-cell processes: evidence for endocrine release of somatostatin. Am J Physiol. 1985 Nov;249(5 Pt 1):G599–G606. doi: 10.1152/ajpgi.1985.249.5.G599. [DOI] [PubMed] [Google Scholar]
- Blom H., Erikoinen T. Trophic effect of pentagastrin on normal and regenerating parietal cells. A light and electron microscopic study in rats. Gastroenterology. 1984 Sep;87(3):537–541. [PubMed] [Google Scholar]
- Bolton J. P., Cohen M. M. Effect of repeated aspirin administration on the gastric mucosal barrier and cell turnover. J Surg Res. 1977 Oct;23(4):251–256. doi: 10.1016/0022-4804(77)90173-1. [DOI] [PubMed] [Google Scholar]
- Casteleyn P. P., Dubrasquet M., Willems G. Opposite effects of gastrin on cell proliferation in the antrum and other parts of the upper-gastrointestinal tract in the rat. Am J Dig Dis. 1977 Sep;22(9):798–804. doi: 10.1007/BF01694510. [DOI] [PubMed] [Google Scholar]
- Crean G. P., Marshall M. W., Rumsey R. D. Parietal cell hyperplasia induced by the administration of pentagastrin (ICI 50,123) to rats. Gastroenterology. 1969 Aug;57(2):147–155. [PubMed] [Google Scholar]
- Deregnaucourt J., Code C. F. Increased resistance of the gastric mucosal barrier to barrier breakers in the rat. Gastroenterology. 1979 Aug;77(2):309–312. [PubMed] [Google Scholar]
- Eastwood G. L., Quimby G. F. Effect of chronic aspirin ingestion on epithelial proliferation in rat fundus, antrum, and duodenum. Gastroenterology. 1982 May;82(5 Pt 1):852–856. [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Dobbs S. M. Gastric adaptation occurs with aspirin administration in man. Dig Dis Sci. 1983 Jan;28(1):1–6. doi: 10.1007/BF01393353. [DOI] [PubMed] [Google Scholar]
- Hinsull S. M., Bellamy D. Effect of repeated colloidal bismuth subcitrate treatment on the response of the rat gastric mucosa to the presence of luminal ethanol. Gut. 1990 Apr;31(4):389–396. doi: 10.1136/gut.31.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey K. J., Tarnawski A., Stachura J., Werner H., Mach T., Burks M. The induction of gastric mucosal tolerance to alcohol by chronic administration. J Lab Clin Med. 1980 Nov;96(5):922–932. [PubMed] [Google Scholar]
- Johnson L. R. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977 Apr;72(4 PT2):788–792. [PubMed] [Google Scholar]
- Karam S. M., Forte J. G. Inhibiting gastric H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am J Physiol. 1994 Apr;266(4 Pt 1):G745–G758. doi: 10.1152/ajpgi.1994.266.4.G745. [DOI] [PubMed] [Google Scholar]
- Karam S. M., Leblond C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993 Jun;236(2):259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- Karam S. M., Leblond C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993 Jun;236(2):280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- Karam S. M., Leblond C. P. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993 Jun;236(2):297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- Karam S. M. New insights into the stem cells and the precursors of the gastric epithelium. Nutrition. 1995 Sep-Oct;11(5 Suppl):607–613. [PubMed] [Google Scholar]
- Karasawa H., Tani N., Miwa T. The effect of omeprazole on ultrastructural changes in gastric parietal cells. Gastroenterol Jpn. 1988 Feb;23(1):1–8. doi: 10.1007/BF02918848. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Kantani-Matsumoto A., Takeoka Y. Epithelial cell proliferation and differentiation in the gastric mucosa: comparisons between histogenetic and cell renewal processes. Prog Clin Biol Res. 1989;295:309–316. [PubMed] [Google Scholar]
- Lacy E. R., Cowart K. S., Hund P., 3rd Effects of chronic superficial injury on the rat gastric mucosa. Gastroenterology. 1992 Oct;103(4):1179–1191. doi: 10.1016/0016-5085(92)91502-u. [DOI] [PubMed] [Google Scholar]
- Lacy E. R. Gastric mucosal resistance to a repeated ethanol insult. Scand J Gastroenterol Suppl. 1985;110:63–72. doi: 10.3109/00365528509095834. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Kuwayama H., Cowart K. S., King J. S., Deutz A. H., Sistrunk S. A rapid, accurate, immunohistochemical method to label proliferating cells in the digestive tract. A comparison with tritiated thymidine. Gastroenterology. 1991 Jan;100(1):259–262. doi: 10.1016/0016-5085(91)90610-w. [DOI] [PubMed] [Google Scholar]
- Lee E. R., Leblond C. P. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985 Mar;172(3):205–224. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- Lehy T., Dubrasquet M., Bonfils S. Effect of somatostatin on normal and gastric-stimulated cell proliferation in the gastric and intestinal mucosae of the rat. Digestion. 1979;19(2):99–109. doi: 10.1159/000198330. [DOI] [PubMed] [Google Scholar]
- Lehy T., Dubrasquet M., Brazeau P., Bonfils S. Inhibitory effect of prolonged administration of long-acting somatostatin on gastrin-stimulated fundic epithelial cell growth in the rat. Digestion. 1982;24(4):246–255. doi: 10.1159/000198804. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970 Jul 24;169(3943):385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- Mayston P. D., Barrowman J. A. Influence of chronic administration of pentagastrin on the pancreas in hypophysectomized rats. Gastroenterology. 1973 Mar;64(3):391–399. [PubMed] [Google Scholar]
- Neuburger P., Lewin M., de Recherche C., Bonfils S. Parietal and chief cell populations in four cases of the Zollinger-Ellison syndrome. Gastroenterology. 1972 Dec;63(6):937–942. [PubMed] [Google Scholar]
- Scheurer U. C., Schlegel J. F., Kelly D. G., Code C. F. Chronic bile exposure increases resistance of canine gastric mucosa to bile. Scand J Gastroenterol Suppl. 1981;67:205–210. [PubMed] [Google Scholar]
- Silen W. Experimental models of gastric ulceration and injury. Am J Physiol. 1988 Oct;255(4 Pt 1):G395–G402. doi: 10.1152/ajpgi.1988.255.4.G395. [DOI] [PubMed] [Google Scholar]
- Smolka A., Alverson L., Fritz R., Swiger K., Swiger R. Gastric H,K-ATPase topography: amino acids 888-907 are cytoplasmic. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1356–1364. doi: 10.1016/s0006-291x(05)81345-2. [DOI] [PubMed] [Google Scholar]
- St John D. J., Yeomans N. D., McDermott F. T., De Boer W. G. Adaptation of the gastric mucosa to repeated administration of aspirin in the rat. Am J Dig Dis. 1973 Oct;18(10):881–885. doi: 10.1007/BF01073339. [DOI] [PubMed] [Google Scholar]
- Walsh J. H., Grossman M. I. Gastrin (first of two parts). N Engl J Med. 1975 Jun 19;292(25):1324–1334. doi: 10.1056/NEJM197506192922505. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]





