Abstract
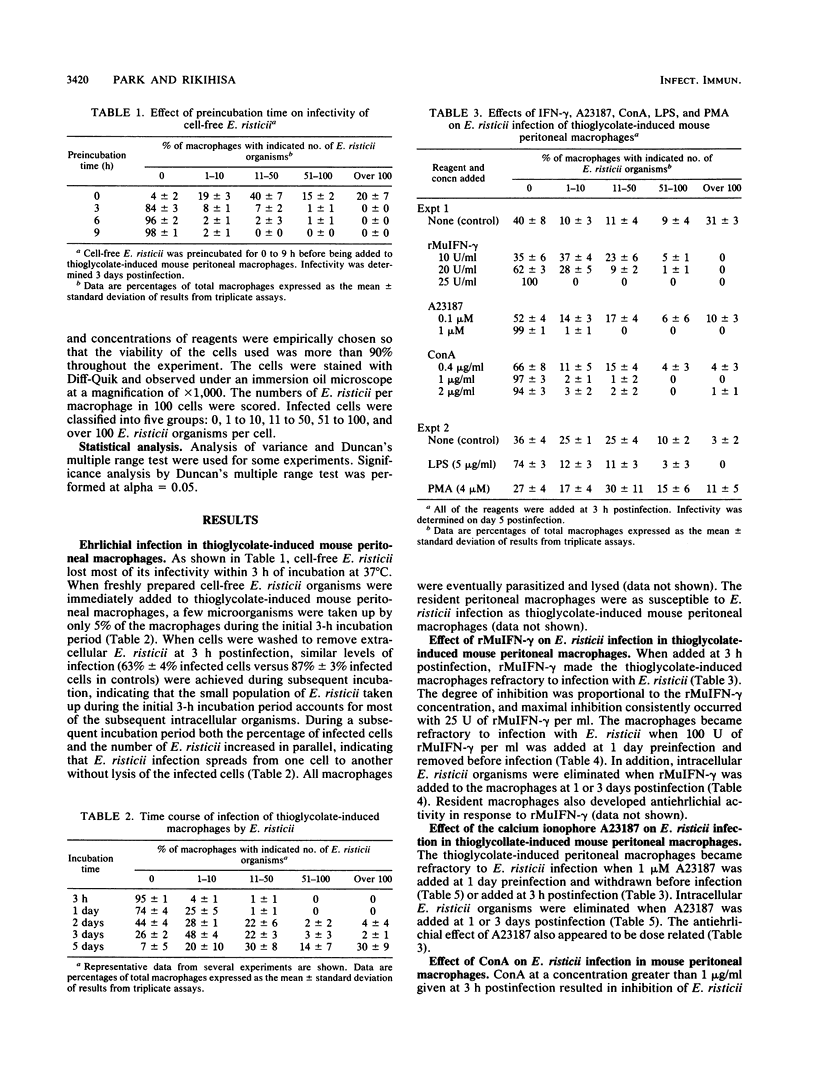
Ehrlichia risticii incubated with mouse peritoneal macrophages elicited with thioglycolate broth survived and replicated, thereby allowing examination of the effects of several immunopotentiating agents. Treatment of the macrophages with recombinant murine gamma interferon (rMuIFN-gamma) in vitro at 1 day before or 3 h after infection made the macrophages resistant to infection with E. risticii, and macrophages treated with rMuIFN-gamma at 1 to 3 days after infection developed the capacity to eradicate intracellular E. risticii. Similar effects were seen with macrophages treated with the Ca2+ ionophore A23187 before or after E. risticii infection in vitro. Concanavalin A treatment before or 3 h after infection caused the macrophages to become resistant to infection with E. risticii but could confer neither ehrlichiacidal nor ehrlichiastic activity to them once infection had been established for more than 1 day. Bacterial products such as lipopolysaccharide and muramyl dipeptide were less or not at all effective, respectively, in conferring antiehrlichial activity to macrophages. Finally, protein kinase C activator, phorbol myristate acetate, and recombinant tumor necrosis factor did not induce any antiehrlichial activity in macrophages when the macrophages were treated either before or after infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsinde J., Fernández B., Diez E. Regulation of arachidonic acid release in mouse peritoneal macrophages. The role of extracellular calcium and protein kinase C. J Immunol. 1990 Jun 1;144(11):4298–4304. [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Blumenthal E. J., Roberts W. K., Vasil A., Talmage D. W. Macrophage activation: dissociation of cytotoxic activity from Ia-A antigen expression. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2031–2035. doi: 10.1073/pnas.80.7.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin J. M., Borden E. C., Byrne G. I. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989 Jun;9(3):329–337. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chung T., Kim Y. B. Two distinct cytolytic mechanisms of macrophages and monocytes activated by phorbol myristate acetate. J Leukoc Biol. 1988 Nov;44(5):329–336. doi: 10.1002/jlb.44.5.329. [DOI] [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- Dorio R. J., Nelson J., Forman H. J. A dual role for calcium in regulation of superoxide generation by stimulated rat alveolar macrophages. Biochim Biophys Acta. 1987 Apr 22;928(2):137–143. doi: 10.1016/0167-4889(87)90114-5. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A. Mechanism of calcium ionophore A23187-induced priming of bone marrow-derived macrophages for tumor cell killing: relationship to priming by interferon. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5959–5962. doi: 10.1073/pnas.82.17.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerich B., Small G. J., Pennington J. E. Relation of cytosolic calcium to the microbicidal activation of blood monocytes by recombinant gamma interferon. J Infect Dis. 1986 Nov;154(5):770–777. doi: 10.1093/infdis/154.5.770. [DOI] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phorbol myristate acetate stimulates phagosome-lysosome fusion in mouse macrophages. J Exp Med. 1981 Jul 1;154(1):101–111. doi: 10.1084/jem.154.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrado L. J., Lewis M. G., Mathes L. E., Olsen R. G. Suppression of in vitro neutrophil function by feline leukaemia virus (FeLV) and purified FeLV-p15E. J Gen Virol. 1987 Feb;68(Pt 2):507–513. doi: 10.1099/0022-1317-68-2-507. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Minakuchi R., Tanaka Y., Hirohata K. The effect of N-(2-carboxyphenyl)-4-chloroantranilic acid disodium salt (CCA) on concanavalin A-induced superoxide anion release in human mononuclear phagocytes. Clin Exp Rheumatol. 1986 Jul-Sep;4(3):243–247. [PubMed] [Google Scholar]
- McLeish K. R., Dean W. L., Wellhausen S. R., Stelzer G. T. Role of intracellular calcium in priming of human peripheral blood monocytes by bacterial lipopolysaccharide. Inflammation. 1989 Dec;13(6):681–692. doi: 10.1007/BF00914312. [DOI] [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Oster C. N., James S. L., Meltzer M. S. Activation of macrophages to kill rickettsiae and Leishmania: dissociation of intracellular microbicidal activities and extracellular destruction of neoplastic and helminth targets. Contemp Top Immunobiol. 1984;13:147–170. doi: 10.1007/978-1-4757-1445-6_8. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Johnson G. C., Burger C. J. Reduced immune responsiveness and lymphoid depletion in mice infected with Ehrlichia risticii. Infect Immun. 1987 Sep;55(9):2215–2222. doi: 10.1128/iai.55.9.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991 Jul;4(3):286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Wedner H. J., Leung M. K., Parker C. W. Effect of serotonin (5-HT) and other monoamines on murine macrophages: modulation of interferon-gamma induced phagocytosis. J Immunol. 1987 Jun 15;138(12):4360–4365. [PubMed] [Google Scholar]
- Torres B. A., Johnson H. M. Lipopolysaccharide and polyribonucleotide activation of macrophages: implications for a natural triggering signal in tumor cell killing. Biochem Biophys Res Commun. 1985 Aug 30;131(1):395–401. doi: 10.1016/0006-291x(85)91815-7. [DOI] [PubMed] [Google Scholar]
- Weiel J. E., Adams D. O., Hamilton T. A. Biochemical models of gamma-interferon action: altered expression of transferrin receptors on murine peritoneal macrophages after treatment in vitro with PMA or A23187. J Immunol. 1985 Jan;134(1):293–298. [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Williams J. C., Dasch G. A., Kang Y. H. Energy metabolism of monocytic Ehrlichia. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1674–1678. doi: 10.1073/pnas.86.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988 Dec;56(12):3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G. M., de la Maza L. M. Activation of mouse peritoneal macrophages in vitro or in vivo by recombinant murine gamma interferon inhibits the growth of Chlamydia trachomatis serovar L1. Infect Immun. 1988 Dec;56(12):3322–3325. doi: 10.1128/iai.56.12.3322-3325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



