Abstract
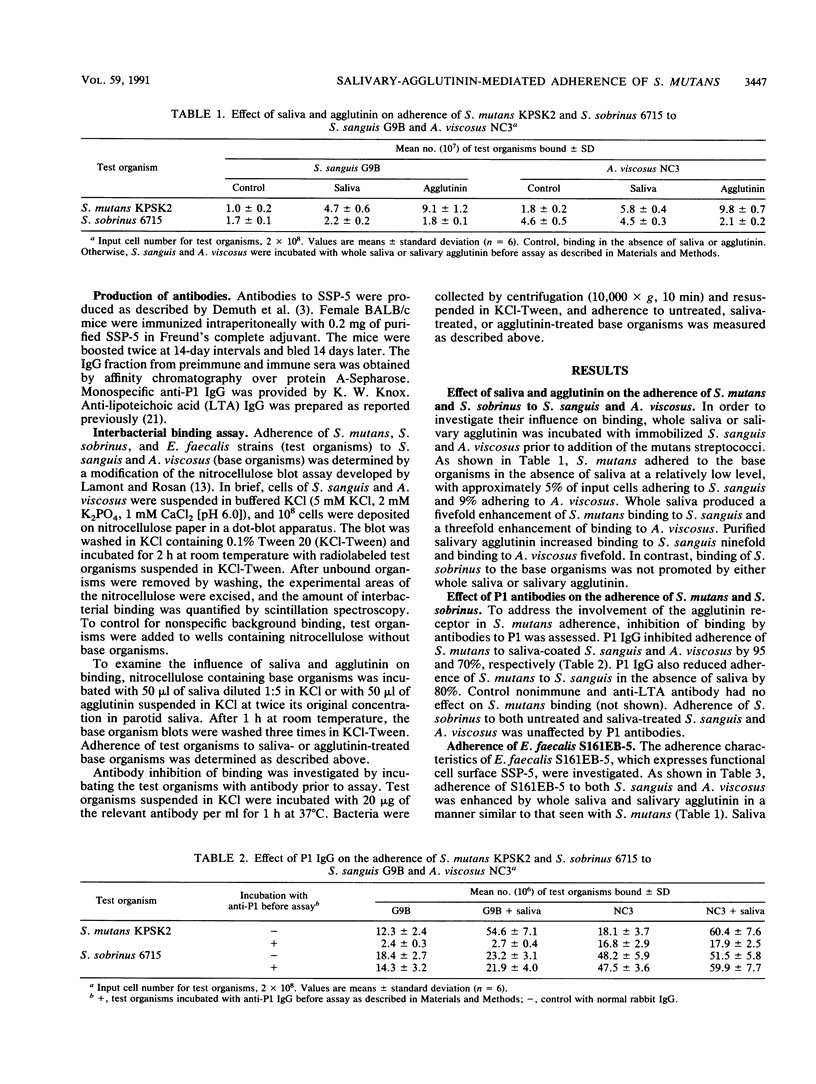
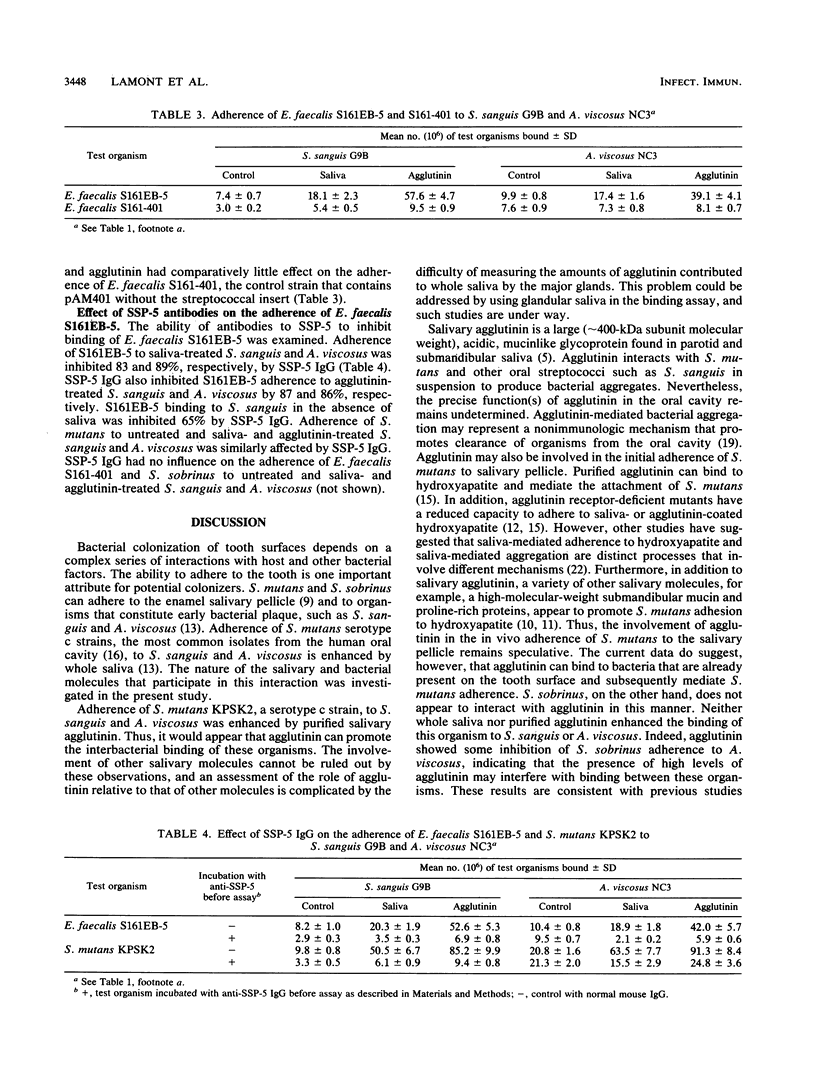
Interspecies binding is important in the colonization of the oral cavity by bacteria. Streptococcus mutans can adhere to other plaque bacteria, such as Streptococcus sanguis and Actinomyces viscosus, and this adherence is enhanced by saliva. The salivary and bacterial molecules that mediate this interaction were investigated. Salivary agglutinin, a mucinlike glycoprotein known to mediate the aggregation of many oral streptococci in vitro, was found to mediate the adherence of S. mutans to S. sanguis or A. viscosus. Adherence of S. mutans to saliva- or agglutinin-coated S. sanguis and A. viscosus was inhibited by antibodies to the bacterial agglutinin receptor. Expression of the S. sanguis receptor (SSP-5) gene in Enterococcus faecalis increased adhesion of this organism to saliva- or agglutinin-coated S. sanguis and A. viscosus. This interaction could be inhibited by antibodies to the agglutinin receptor. The results suggest that salivary agglutinin can promote adherence of S. mutans to S. sanguis and A. viscosus through interactions with the agglutinin receptor on S. mutans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu J. P., Dabbous M. K. Interaction of salivary fibronectin with oral streptococci. J Dent Res. 1986 Aug;65(8):1094–1100. doi: 10.1177/00220345860650081001. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd 1984 Kreshover lecture. Genetic analysis of Streptococcus mutans virulence and prospects for an anticaries vaccine. J Dent Res. 1986 Aug;65(8):1034–1045. doi: 10.1177/00220345860650080101. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Berthold P., Leboy P. S., Golub E. E., Davis C. A., Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989 May;57(5):1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Davis C. A., Corner A. M., Lamont R. J., Leboy P. S., Malamud D. Cloning and expression of a Streptococcus sanguis surface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988 Sep;56(9):2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Demuth D. R., Lammey M. S., Huck M., Lally E. T., Malamud D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb Pathog. 1990 Sep;9(3):199–211. doi: 10.1016/0882-4010(90)90022-i. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Russell R. R. The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(10):751–757. doi: 10.1016/0003-9969(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Ericson T., Pruitt K., Wedel H. The reaction of salivary substances with bacteria. J Oral Pathol. 1975 Dec;4(6):307–323. doi: 10.1111/j.1600-0714.1975.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Adsorbed salivary acidic proline-rich proteins contribute to the adhesion of Streptococcus mutans JBP to apatitic surfaces. J Dent Res. 1989 Sep;68(9):1303–1307. doi: 10.1177/00220345890680090201. [DOI] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989 Dec;57(12):3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Okahashi N., Takahashi I., Kanamoto T., Asakawa H., Iwaki M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect Immun. 1990 Feb;58(2):289–296. doi: 10.1128/iai.58.2.289-296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. J., Rosan B. Adherence of mutans streptococci to other oral bacteria. Infect Immun. 1990 Jun;58(6):1738–1743. doi: 10.1128/iai.58.6.1738-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Erdos G. W., Piacentini D. A., Ayakawa G. Y., Crowley P. J., Bleiweis A. S. Construction and characterization of isogenic mutants of Streptococcus mutans deficient in major surface protein antigen P1 (I/II). Infect Immun. 1989 Nov;57(11):3306–3313. doi: 10.1128/iai.57.11.3306-3313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay B. J., Denepitiya L., Iacono V. J., Krost S. B., Pollock J. J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984 Jun;44(3):695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D., Appelbaum B., Kline R., Golub E. E. Bacterial aggregating activity in human saliva: comparisons of bacterial species and strains. Infect Immun. 1981 Mar;31(3):1003–1006. doi: 10.1128/iai.31.3.1003-1006.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel I. D. Nonimmunologic aspects of caries resistance. J Dent Res. 1976 Apr;55(Spec No):C22–C31. doi: 10.1177/002203457605500316011. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundegren J. Calcium-dependent salivary agglutinin with reactivity to various oral bacterial species. Infect Immun. 1986 Jul;53(1):173–178. doi: 10.1128/iai.53.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Distribution of cross-reactive antigens A and B in Streptococcus mutans and other oral streptococci. J Gen Microbiol. 1980 Jun;118(2):383–388. doi: 10.1099/00221287-118-2-383. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Homology between surface protein antigen genes of Streptococcus sobrinus and Streptococcus mutans. FEBS Lett. 1989 Jun 5;249(2):383–388. doi: 10.1016/0014-5793(89)80664-7. [DOI] [PubMed] [Google Scholar]
- Wirth R., An F. Y., Clewell D. B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986 Mar;165(3):831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



