Abstract
Six-micron paraffin sections of paraformaldehyde-fixed specimens of 24 ovarian benign and neoplastic specimens were assayed for tumor cell-specific oncogene expression by a sensitive, quantitative in situ hybridization technique with probes for 17 oncogenes, beta-actin, and E. coli beta-lactamase. In the benign, borderline, and invasive adenocarcinomas, multiple oncogenes, including neu, fes, fms, Ha-ras, trk, c-myc, fos, and PDGF-A chains, were expressed at significant levels relative to a housekeeping gene (beta-actin). In the mixed-Mullerian tumors, a rather different pattern of oncogene expression was observed, characterized primarily by expression of sis (PDGF-B chain). For the adenocarcinomas, statistical analysis demonstrated that expression of several genes (fms, neu, PDGF-A) was closely linked to others (c-fos, c-myc) known to have important roles in the control of cell proliferation, but only one gene, fms, correlated very strongly with clinicopathologic features (high FIGO histologic grade and high FIGO clinical stage) predictive of aggressive clinical behavior and poor outcome. The authors discuss the role that tumor epithelial cell expression of the fms gene product might play in the auto- and paracrine control of growth and dissemination of ovarian adenocarcinomas.
Full text
PDF

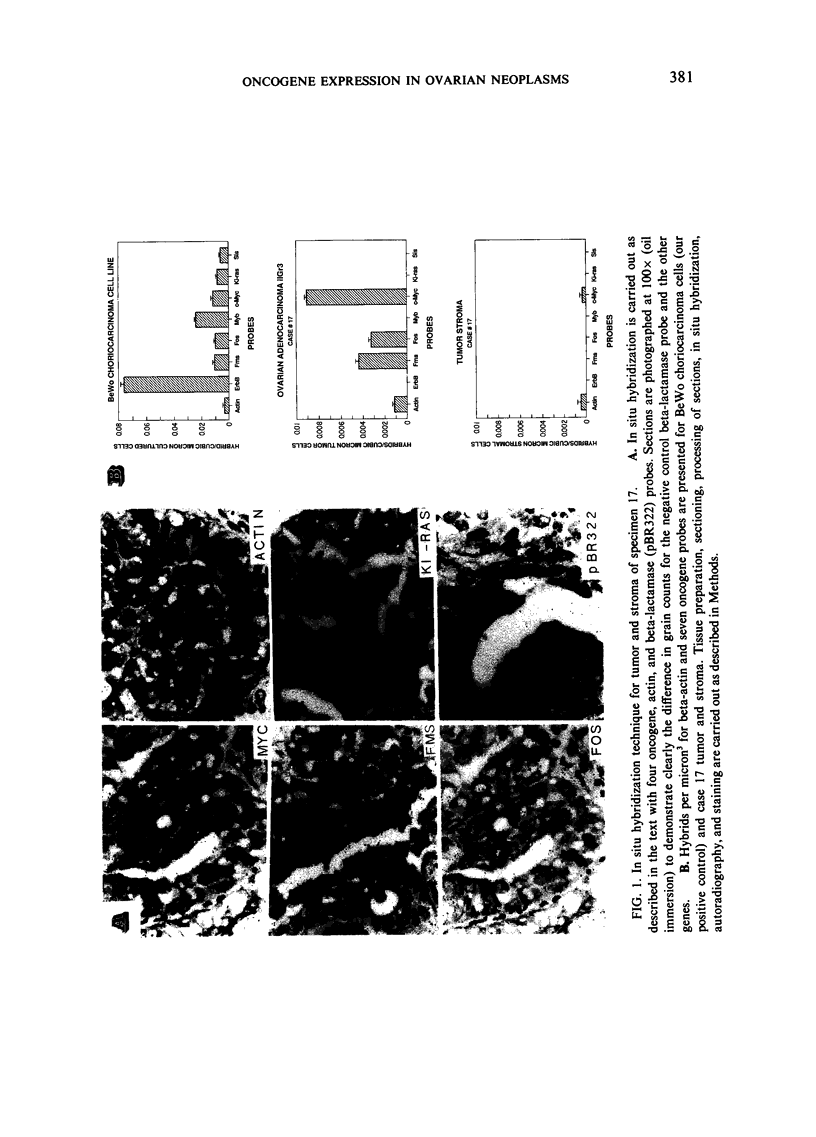
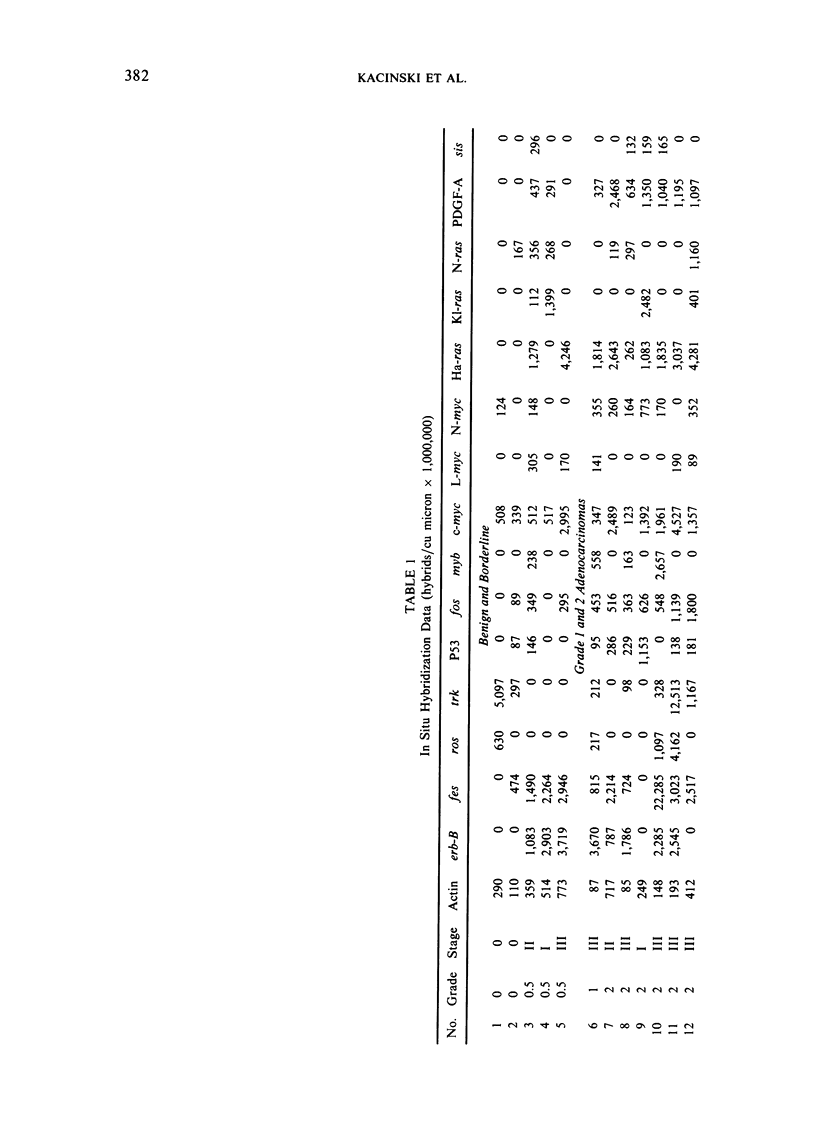


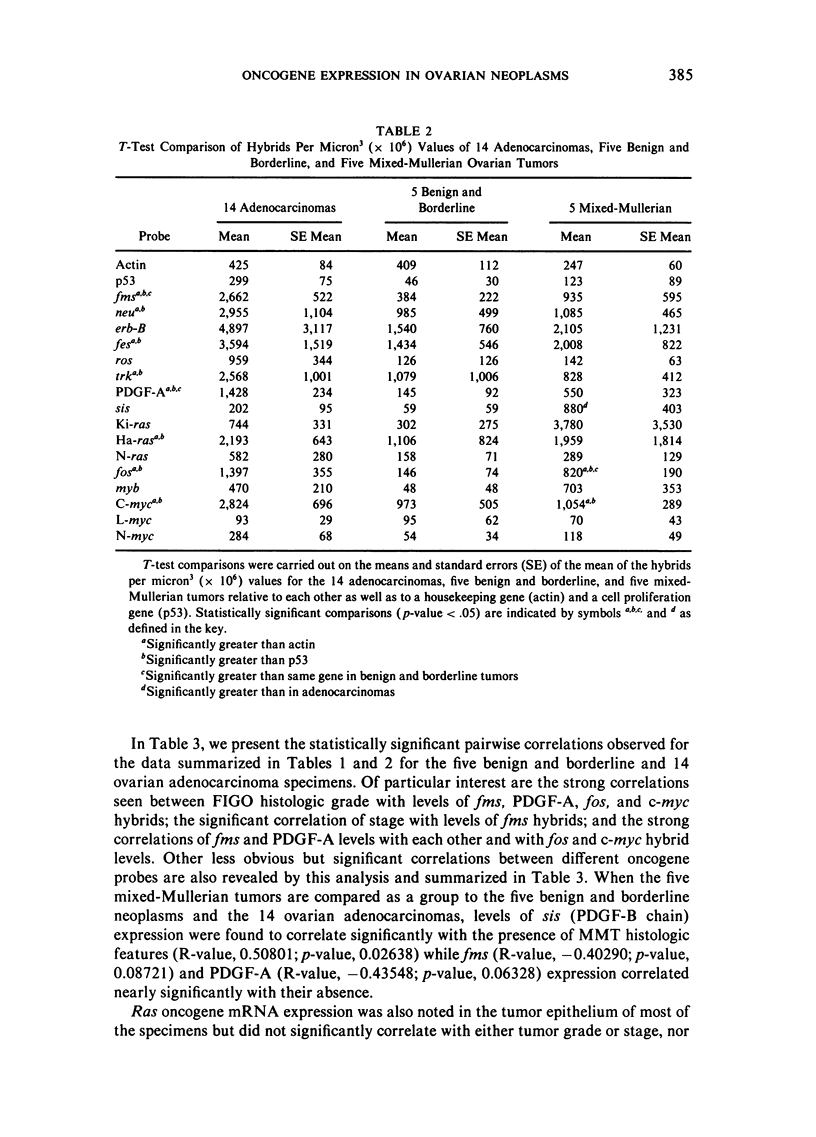
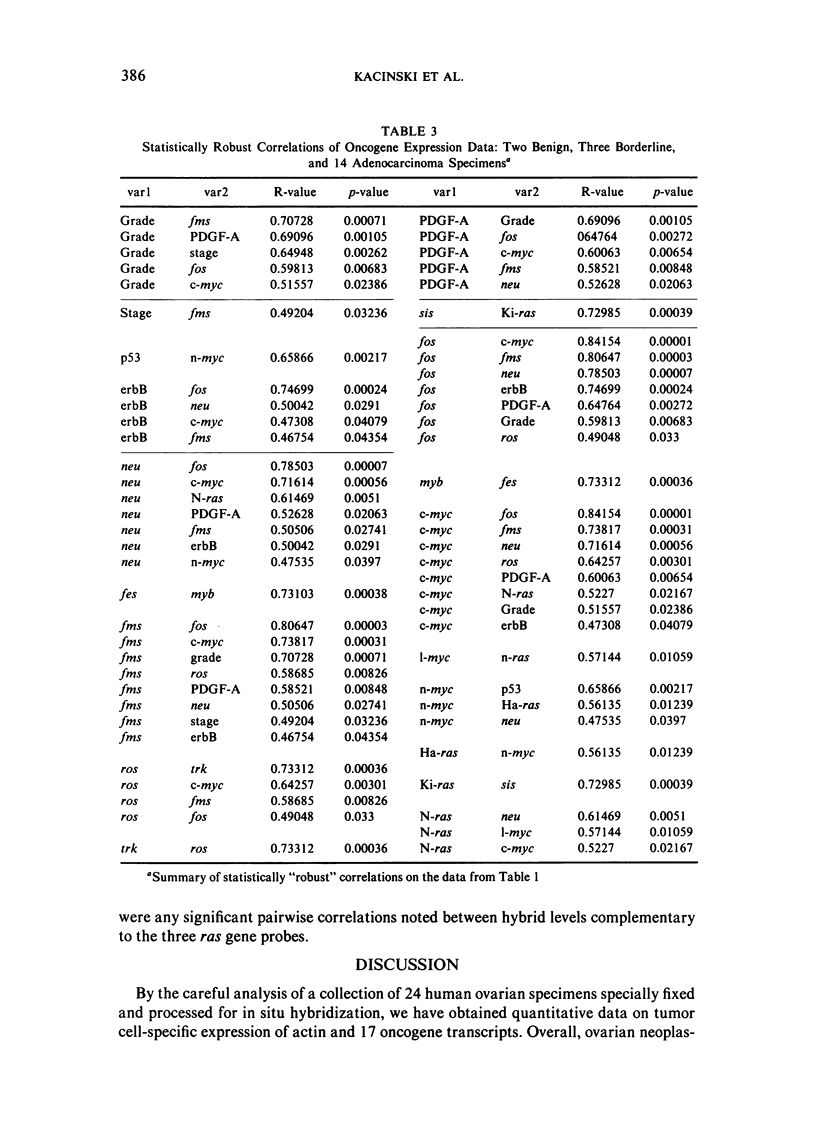






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K., Boyer H. W. Tetracycline resistance determined by pBR322 is mediated by one polypeptide. Gene. 1983 Dec;26(2-3):197–203. doi: 10.1016/0378-1119(83)90190-7. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Carney W. P., Hamer P., Petit D., Wolfe H., Cooper G., Lefebvre M., Rabin H. A monoclonal antibody reactive with an activated ras protein expressing valine at position 12. J Cell Biochem. 1986;32(3):207–214. doi: 10.1002/jcb.240320307. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Curran T., MacConnell W. P., van Straaten F., Verma I. M. Structure of the FBJ murine osteosarcoma virus genome: molecular cloning of its associated helper virus and the cellular homolog of the v-fos gene from mouse and human cells. Mol Cell Biol. 1983 May;3(5):914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Furth M. E., Scolnick E. M. Mouse cells contain two distinct ras gene mRNA species that can be translated into a p21 onc protein. Mol Cell Biol. 1982 Nov;2(11):1339–1345. doi: 10.1128/mcb.2.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Godard C., Jones K. W. Detection of AKR MuLV-specific RNA in AKR mouse cells by in situ hybridization. Nucleic Acids Res. 1979 Jun 25;6(8):2849–2861. doi: 10.1093/nar/6.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Redmond S., Wade-Evans A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucleic Acids Res. 1984 Jul 25;12(14):5609–5626. doi: 10.1093/nar/12.14.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Kohorn E. I., Bloodgood R. S., Donahue J., Donofrio L., Edwards R., Schwartz P. E., Chambers J. T. High level expression of fms proto-oncogene mRNA is observed in clinically aggressive human endometrial adenocarcinomas. Int J Radiat Oncol Biol Phys. 1988 Oct;15(4):823–829. doi: 10.1016/0360-3016(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Stanley E. R., Carter D., Chambers J. T., Chambers S. K., Kohorn E. I., Schwartz P. E. Circulating levels of CSF-1 (M-CSF) a lymphohematopoietic cytokine may be a useful marker of disease status in patients with malignant ovarian neoplasms. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):159–164. doi: 10.1016/0360-3016(89)90383-0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Legouy E., DePinho R. A., Nisen P. D., Smith R. K., Gee C. E., Alt F. W. Human N-myc is closely related in organization and nucleotide sequence to c-myc. Nature. 1986 Jan 2;319(6048):73–77. doi: 10.1038/319073a0. [DOI] [PubMed] [Google Scholar]
- Kraus M. H., Pierce J. H., Fleming T. P., Robbins K. C., Di Fiore P. P., Aaronson S. A. Mechanisms by which genes encoding growth factors and growth factor receptors contribute to malignant transformation. Ann N Y Acad Sci. 1988;551:320–336. doi: 10.1111/j.1749-6632.1988.tb22358.x. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Wang L. H., Shibuya M. Human c-ros-1 gene homologous to the v-ros sequence of UR2 sarcoma virus encodes for a transmembrane receptorlike molecule. Mol Cell Biol. 1986 Aug;6(8):3000–3004. doi: 10.1128/mcb.6.8.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Tremblay J. M., Adamson E. D., Verma I. M. Tissue and cell type-specific expression of two human c-onc genes. Nature. 1983 Aug 4;304(5925):454–456. doi: 10.1038/304454a0. [DOI] [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Ohuchi N., Hand P. H., Merlo G., Fujita J., Mariani-Costantini R., Thor A., Nose M., Callahan R., Schlom J. Enhanced expression of c-Ha-ras p21 in human stomach adenocarcinomas defined by immunoassays using monoclonal antibodies and in situ hybridization. Cancer Res. 1987 Mar 1;47(5):1413–1420. [PubMed] [Google Scholar]
- Ozols R. F., Garvin A. J., Costa J., Simon R. M., Young R. C. Advanced ovarian cancer: correlation of histologic grade with response to therapy and survival. Cancer. 1980 Feb;45(3):572–581. doi: 10.1002/1097-0142(19800201)45:3<572::aid-cncr2820450325>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968 Jul;28(7):1231–1236. [PubMed] [Google Scholar]
- Rajavashisth T. B., Eng R., Shadduck R. K., Waheed A., Ben-Avram C. M., Shively J. E., Lusis A. J. Cloning and tissue-specific expression of mouse macrophage colony-stimulating factor mRNA. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1157–1161. doi: 10.1073/pnas.84.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Sacca R., Furman W. L., Roussel M. F., Holt J. T., Nienhuis A. W., Stanley E. R., Sherr C. J. Expression of the human c-fms proto-oncogene product (colony-stimulating factor-1 receptor) on peripheral blood mononuclear cells and choriocarcinoma cell lines. J Clin Invest. 1986 Jun;77(6):1740–1746. doi: 10.1172/JCI112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Scully R. E. Ovarian tumors. A review. Am J Pathol. 1977 Jun;87(3):686–720. [PMC free article] [PubMed] [Google Scholar]
- Scully R. E. World Health Organization classification and nomenclature of ovarian cancer. Natl Cancer Inst Monogr. 1975 Oct;42:5–7. [PubMed] [Google Scholar]
- Seemayer T. A., Cavenee W. K. Molecular mechanisms of oncogenesis. Lab Invest. 1989 May;60(5):585–599. [PubMed] [Google Scholar]
- Serunian L. A., Cantley L. C. Growth factor and oncogene influences on cell growth regulation. Ann N Y Acad Sci. 1988;551:309–319. doi: 10.1111/j.1749-6632.1988.tb22357.x. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Perucho M., Wigler M. Isolation and preliminary characterization of the transforming gene of a human neuroblastoma cell line. Proc Natl Acad Sci U S A. 1983 Jan;80(2):383–387. doi: 10.1073/pnas.80.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Suard Y., Perucho M., Li Y., Kamata T., Feramisco J., Stavnezer E., Fogh J., Wigler M. H. Three human transforming genes are related to the viral ras oncogenes. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2112–2116. doi: 10.1073/pnas.80.8.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. Action of the colony-stimulating factor, CSF-1. Ciba Found Symp. 1986;118:29–41. doi: 10.1002/9780470720998.ch3. [DOI] [PubMed] [Google Scholar]
- Thor A., Horan Hand P., Wunderlich D., Caruso A., Muraro R., Schlom J. Monoclonal antibodies define differential ras gene expression in malignant and benign colonic diseases. Nature. 1984 Oct 11;311(5986):562–565. doi: 10.1038/311562a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Finkel G., Lundy J., Hand P., Thor A., Schlom J. Expression of ras oncogene p21 in prostate cancer. N Engl J Med. 1986 Jan 16;314(3):133–137. doi: 10.1056/NEJM198601163140301. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]
- Walker C., Nettesheim P., Barrett J. C., Gilmer T. M. Expression of a fms-related oncogene in carcinogen-induced neoplastic epithelial cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1804–1808. doi: 10.1073/pnas.84.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee L. D., Kacinski B. M., Carter D. Oncogene structure, function, and expression in breast cancer. Semin Diagn Pathol. 1989 May;6(2):110–125. [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]




