Abstract
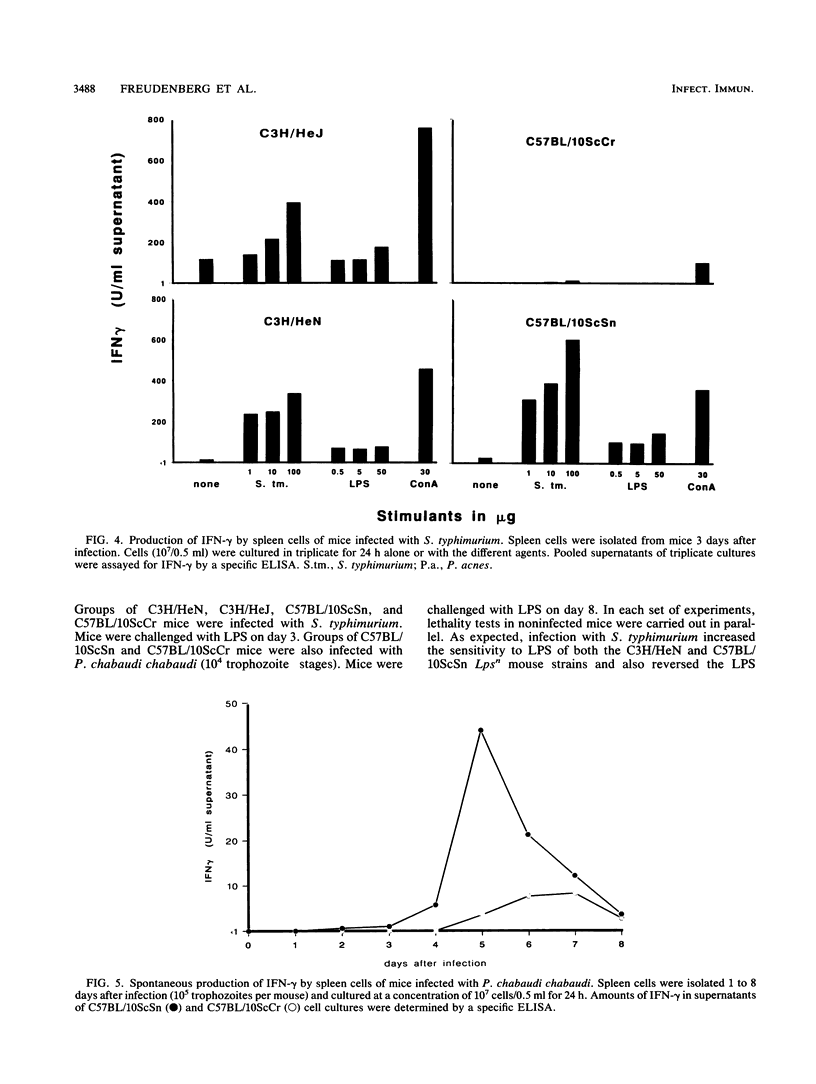
The production of gamma interferon (IFN-gamma) in response to infection and to a number of other agents was compared in Lpsn (C3H/HeN and C57BL/10ScSn) and Lpsd (C3H/HeJ and C57BL/10ScCr) mouse strains. Large differences in IFN-gamma production were observed between C57BL/10ScCr mice and the other mouse strains. With the exception of C57BL/10ScCr, all mouse strains, including C3H/HeJ, exhibited transient levels of IFN-gamma during infection with Salmonella typhimurium. Spleen cells of these mice, explanted on day 3 of infection, produced in vitro IFN-gamma spontaneously; this production was enhanced considerably by heat-killed S. typhimurium, heat-killed Propionibacterium acnes, concanavalin A (ConA), or lipopolysaccharide (LPS). These stimuli, except for LPS, also induced IFN-gamma production in cultures of normal spleen cells from noninfected animals. In contrast, C57BL/10ScCr mice produced no IFN-gamma following infection with S. typhimurium. Also, spleen cells of these mice, explanted on day 3 of infection, exhibited no spontaneous IFN-gamma production. A marginal response was obtained by additional stimulation of the cells with killed S. typhimurium, and a moderate response was obtained with ConA. Normal spleen cells from noninfected C57BL/10ScCr mice showed no IFN-gamma response to killed S. typhimurium, killed P. acnes, or LPS and only a low response to ConA. Impaired IFN-gamma production in C57BL/10ScCr mice was also evident during infection with Plasmodium chabaudi chabaudi, with which a low IFN-gamma response was seen only occasionally. Also, spleen cells from infected animals (days 2 to 8 after infection) exhibited only a very low level of IFN-gamma production in vitro; however, this production could be enhanced further by ConA. In comparison, C57BL/10ScSn mice infected with P. chabaudi chabaudi produced significant amounts of IFN-gamma. Spleen cells explanted from infected animals produced IFN-gamma spontaneously in vitro; this production was enhanced further by killed P. acnes and ConA. The results showed that in addition to the defect in LPS responsiveness, C57BL/10ScCr mice possess a defect in IFN-gamma production in response to different stimuli. During infection, IFN-gamma production and sensitization to LPS occurred in parallel. Infected Lpsn mice exhibited enhanced sensitivity and infected Lpsd C3H/HeJ mice exhibited reasonable sensitivity to the lethal effects of LPS. Lpsd C57BL/10ScCr mice remained resistant to LPS when infected with S. typhimurium and exhibited only marginal sensitivity when infected with P. chabaudi chabaudi.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Beutler B., Tkacenko V., Milsark I., Krochin N., Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986 Nov 1;164(5):1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. K., Djeu J. Y., Klein T. W., Friedman H., Stewart W. E., 2nd Interferon-gamma induction by lipopolysaccharide: dependence on interleukin 2 and macrophages. J Immunol. 1986 Feb 1;136(3):963–970. [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of a standardized lipopolysaccharide from salmonella abortus equi (Novo-Pyrexal). Zentralbl Bakteriol Orig A. 1979 Apr;243(2-3):226–244. [PubMed] [Google Scholar]
- Gifford G. E., Lohmann-Matthes M. L. Gamma interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J Natl Cancer Inst. 1987 Jan;78(1):121–124. doi: 10.1093/jnci/78.1.121. [DOI] [PubMed] [Google Scholar]
- Handa K., Suzuki R., Matsui H., Shimizu Y., Kumagai K. Natural killer (NK) cells as a responder to interleukin 2 (IL 2). II. IL 2-induced interferon gamma production. J Immunol. 1983 Feb;130(2):988–992. [PubMed] [Google Scholar]
- Huang K. Y., Schultz W. W., Gordon F. B. Interferon induced by Plasmodium berghei. Science. 1968 Oct 4;162(3849):123–124. doi: 10.1126/science.162.3849.123. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Vilcek J., Nussenzweig R. S. Exogenous interferon protects mice against Plasmodium berghei malaria. Nature. 1970 Sep 26;227(5265):1350–1351. doi: 10.1038/2271350a0. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Stanton G. J., Baron S. Relative ability of mitogens to stimulate production of interferon by lymphoid cells and to induce suppression of the in vitro immune response. Proc Soc Exp Biol Med. 1977 Jan;154(1):138–141. [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Lin J. X., Henriksen-DeStefano D., Vilcek J. Bacterial lipopolysaccharide-induced interferon-gamma production: roles of interleukin 1 and interleukin 2. J Immunol. 1986 Jun 15;136(12):4525–4530. [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose L. D., Trejo R., Di Luzio N. R. Impaired endotoxin detoxification as a factor in enhanced endotoxin sensitivity of malaria infected mice. Proc Soc Exp Biol Med. 1971 Jul;137(3):794–797. doi: 10.3181/00379727-137-35669. [DOI] [PubMed] [Google Scholar]
- Marcucci F., Waller M., Kirchner H., Krammer P. Production of immune interferon by murine T-cell clones from long-term cultures. Nature. 1981 May 7;291(5810):79–81. doi: 10.1038/291079a0. [DOI] [PubMed] [Google Scholar]
- Matsumura H., Nakano M. Endotoxin-induced interferon-gamma production in culture cells derived from BCG-infected C3H/HeJ mice. J Immunol. 1988 Jan 15;140(2):494–500. [PubMed] [Google Scholar]
- Matsuura M., Galanos C. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect Immun. 1990 Apr;58(4):935–937. doi: 10.1128/iai.58.4.935-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meding S. J., Cheng S. C., Simon-Haarhaus B., Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990 Nov;58(11):3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Muotiala A., Mäkelä P. H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990 Feb;8(2):135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- Nathan I., Groopman J. E., Quan S. G., Bersch N., Golde D. W. Immune (gamma) interferon produced by a human T-lymphoblast cell line. Nature. 1981 Aug 27;292(5826):842–844. doi: 10.1038/292842a0. [DOI] [PubMed] [Google Scholar]
- Prat M., Gribaudo G., Comoglio P. M., Cavallo G., Landolfo S. Monoclonal antibodies against murine gamma interferon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., ULLMAN G. E., HOFFMAN R. G. Sensitivity of mice to endotoxin after vaccination with BCG (Bacillus Calmette-Guérin). Proc Soc Exp Biol Med. 1958 Oct;99(1):167–169. doi: 10.3181/00379727-99-24282. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Schramek S., Kazar J., Sekeyova Z., Freudenberg M. A., Galanos C. Induction of hyperreactivity to endotoxin in mice by Coxiella burnetii. Infect Immun. 1984 Sep;45(3):713–717. doi: 10.1128/iai.45.3.713-717.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear H. L., Srinivasan R., Nolan T., Ng C. Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol. 1989 Sep 15;143(6):2038–2044. [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Spitalny G. L., Havell E. A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984 May 1;159(5):1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Nowotarski M. Role of interferon-gamma and tumor necrosis factor in host resistance to Plasmodium chabaudi AS. Immunol Lett. 1990 Aug;25(1-3):115–121. doi: 10.1016/0165-2478(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Galanos C., Lüderitz O., Kusumoto S., Shiba T. Mitogenic activities of synthetic lipid A analogs and suppression of mitogenicity of lipid A. Infect Immun. 1984 May;44(2):427–433. doi: 10.1128/iai.44.2.427-433.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Fultz M. J. Lps gene-associated functions. Curr Top Microbiol Immunol. 1988;137:165–170. doi: 10.1007/978-3-642-50059-6_24. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Hansen C. T., Rosenstreich D. L. Characterization of a congenitally LPS-resistant, athymic mouse strain. J Immunol. 1979 Feb;122(2):619–622. [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]



