Abstract
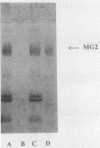
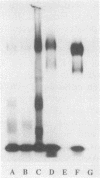
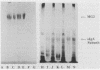
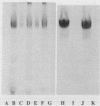
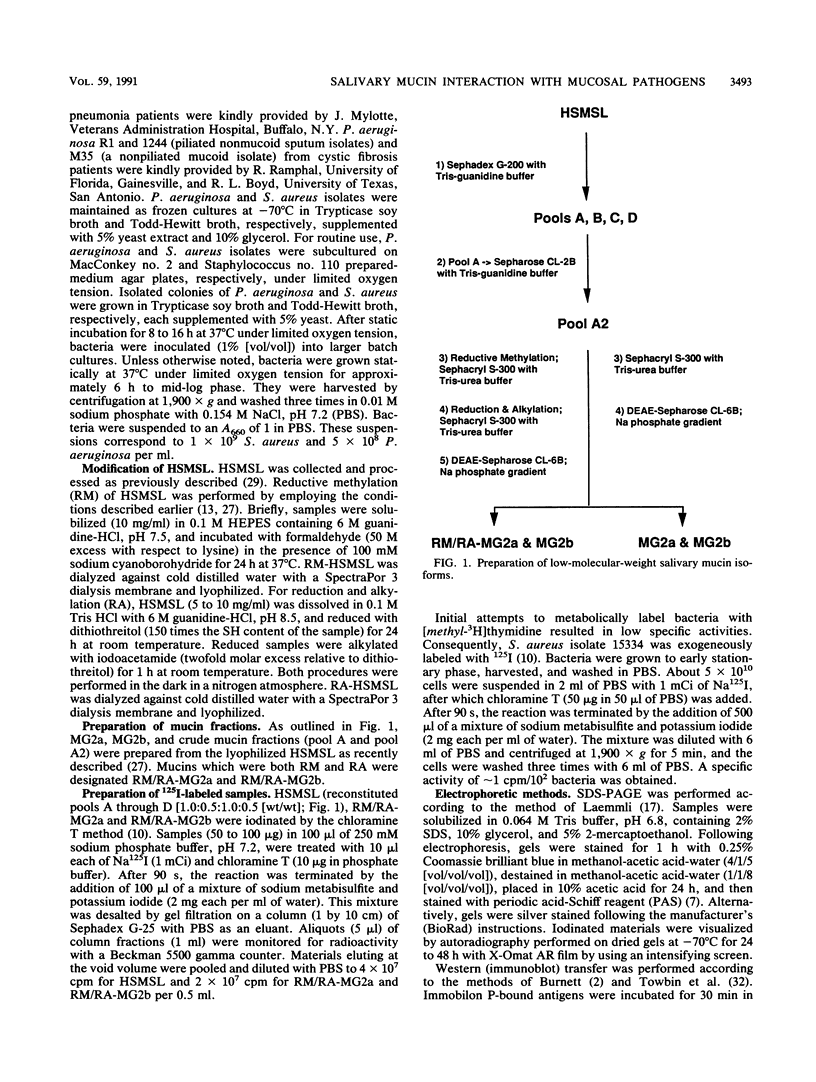
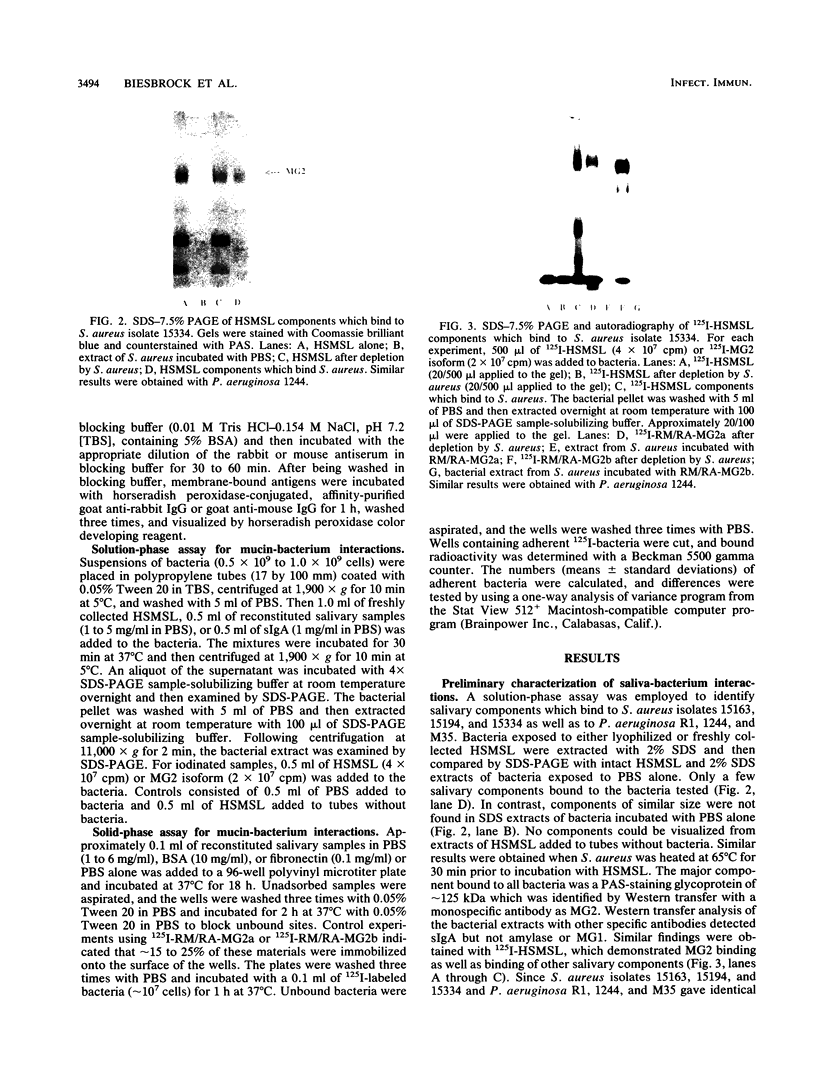
This study examined the interaction of a human salivary low-molecular-weight mucin (MG2) with Staphylococcus aureus and Pseudomonas aeruginosa by using both solution-phase and solid-phase assays. In solution phase, MG2 in human submandibular-sublingual saliva (HSMSL) bound to the bacterial surface; however, the highly purified mucin isoforms (MG2a and MG2b) did not. Mucin binding appeared to be dependent on heterotypic complexing between MG2 and secretory immunoglobulin A (IgA), although other salivary molecules may also be involved. In contrast, in a solid-phase assay in which HSMSL, MG2-containing fractions with secretory IgA, and purified MG2 were immobilized onto a solid surface, there was minimal adherence of S. aureus. The collective results suggest that mucin binding to S. aureus and P. aeruginosa may be predicated on the formation of an MG2-secretory IgA complex. Such interactions may facilitate microbial clearance from the oral cavity and play an important role in preventing colonization of the oral cavity and the respiratory tract by potential pathogens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brack C. M., Reynolds E. C. Characterization of a rat salivary sialoglycoprotein complex which agglutinates Streptococcus mutans. Infect Immun. 1987 May;55(5):1264–1273. doi: 10.1128/iai.55.5.1264-1273.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Beem J. E., Nesbitt W. E., Cisar J. O., Tseng C. C., Levine M. J. Pellicle receptors for Actinomyces viscosus type 1 fimbriae in vitro. Infect Immun. 1989 Oct;57(10):3003–3008. doi: 10.1128/iai.57.10.3003-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. E., Aguirre A., Neiders M. E., Levine M. J., Jones P. C., Reddy M. S., Haar J. G. Immunochemistry and immunogenicity of low molecular weight human salivary mucin. Arch Oral Biol. 1991;36(5):347–356. doi: 10.1016/0003-9969(91)90004-E. [DOI] [PubMed] [Google Scholar]
- Cohen R. E., Aguirre A., Neiders M. E., Levine M. J., Jones P. C., Reddy M. S., Haar J. G. Immunochemistry of high molecular-weight human salivary mucin. Arch Oral Biol. 1990;35(2):127–136. doi: 10.1016/0003-9969(90)90174-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton M. N., Loomis R. E., Levine M. J., Tabak L. A. Masticatory lubrication. The role of carbohydrate in the lubricating property of a salivary glycoprotein-albumin complex. Biochem J. 1985 Sep 15;230(3):817–820. doi: 10.1042/bj2300817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Johanson W. G., Jr, Woods D. E., Chaudhuri T. Association of respiratory tract colonization with adherence of gram-negative bacilli to epithelial cells. J Infect Dis. 1979 Jun;139(6):667–673. doi: 10.1093/infdis/139.6.667. [DOI] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Tumber S. K. Role of sialic acid in saliva-mediated aggregation of Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1987 Oct;55(10):2364–2369. doi: 10.1128/iai.55.10.2364-2369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Tumber S. K. Whole, submandibular, and parotid saliva-mediated aggregation of Pseudomonas aeruginosa in cystic fibrosis. Infect Immun. 1989 Apr;57(4):1299–1304. doi: 10.1128/iai.57.4.1299-1304.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Ofstehage J. C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979 Dec;26(3):1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann R. A., Newman M. G., Kaufman A. K., Le T. V. Oral colonization and susceptibility testing of Pseudomonas aeruginosa oral isolates from cystic fibrosis patients. J Dent Res. 1985 Jan;64(1):54–57. doi: 10.1177/00220345850640011001. [DOI] [PubMed] [Google Scholar]
- Loomis R. E., Prakobphol A., Levine M. J., Reddy M. S., Jones P. C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987 Nov 1;258(2):452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- Magnusson K. E., Stjernström I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology. 1982 Feb;45(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Gisslow M. T. Role of sialic acid in saliva-induced aggregation of Streptococcus sanguis. Infect Immun. 1977 Oct;18(1):35–40. doi: 10.1128/iai.18.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Prakobphol A., Levine M. J., Tabak L. A., Reddy M. S. Purification of a low-molecular-weight, mucin-type glycoprotein from human submandibular-sublingual saliva. Carbohydr Res. 1982 Oct 1;108(1):111–122. doi: 10.1016/s0008-6215(00)81896-0. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Thomas V. L., Ramsay M. A. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun. 1989 Dec;57(12):3735–3742. doi: 10.1128/iai.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Hay D. I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Feb;61(2):397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A., Zielenski J., Mandel I. D. Mucus glycoprotein of human saliva: differences in the associated and covalently bound lipids with caries. Biochim Biophys Acta. 1986 Jun 3;882(1):18–28. doi: 10.1016/0304-4165(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Levine M. J., Cavese J. M., Prakobphol A., Murray P. A., Tabak L. A., Reddy M. S. Adherence of Streptococcus sanguis to salivary mucin bound to glass. J Dent Res. 1982 Dec;61(12):1390–1393. doi: 10.1177/00220345820610120101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti W. M., Trudell R. G., Bentley D. W. Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N Engl J Med. 1978 May 18;298(20):1108–1111. doi: 10.1056/NEJM197805182982002. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke C. A., Cronan S., Goss C., Chadee K., Guerrant R. L. Characterization of binding of Escherichia coli strains which are enteropathogens to small-bowel mucin. Infect Immun. 1990 Mar;58(3):794–800. doi: 10.1128/iai.58.3.794-800.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gibbons R. J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972 Aug 25;177(4050):697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]