Abstract
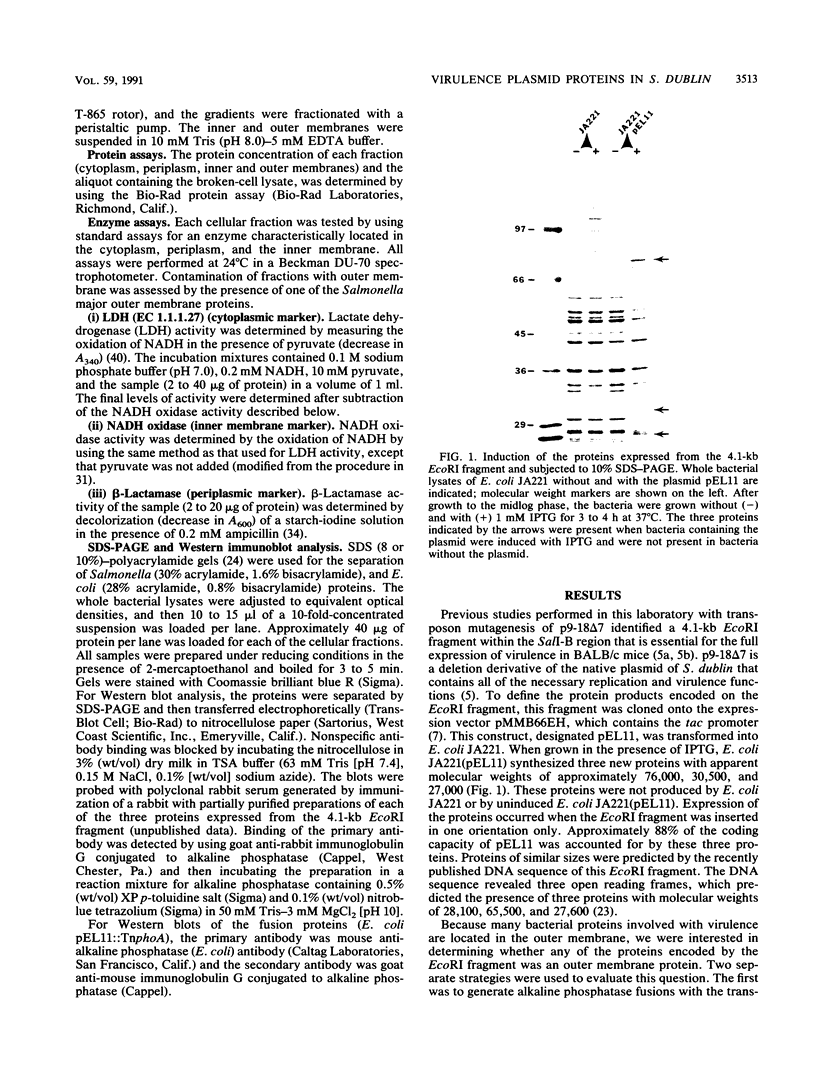
Infection of both cattle and humans with Salmonella dublin can result in septicemia and death. Like many nontyphoid Salmonella species that cause disease, S. dublin contains a cryptic plasmid (pSDL2) that is required for the full expression of virulence. Transposon mutagenesis of pSDL2 defined a 4.1-kb EcoRI region that is necessary for the development of a systemic infection in BALB/c mice. This EcoRI fragment was cloned into an expression vector (pEL11), and three proteins produced from this region with apparent molecular weights of 30,500, 76,000, and 27,000 were identified. Because bacterial proteins that play a role in virulence are often associated with the outer membrane, we were interested in establishing whether the proteins expressed from the EcoRI fragment are located in the membrane. Transposon mutagenesis of pEL11 with TnphoA defined the order of the genes along the fragment and suggested that the proteins may be exported out of the cytoplasm. Sucrose gradient cell fractionation was done to identify the cellular location of each of the three proteins. The 30-kDa protein was identified in the outer membrane fraction, and the 76-kDa protein was located in the cytosolic fraction. The 27-kDa protein was identified in both the cytosolic and the outer membrane fractions. The outer membrane contained less than 10% of the activity of enzymes known to be located in the cytoplasm, periplasm, and inner membrane. Sequence data of the 4.1-kb EcoRI region revealed that both the 30- and the 27-kDa proteins lack a typical signal sequence for export out of the cytoplasm (M. Krause, C. Roudier, J. Fierer, J. Harwood, and D. G. Guiney, Mol. Microbiol. 5:307, 1991). The outer membrane location of these proteins suggests that they may be exported out of the cytoplasm by an unusual mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow P. A., Simpson J. M., Lovell M. A., Binns M. M. Contribution of Salmonella gallinarum large plasmid toward virulence in fowl typhoid. Infect Immun. 1987 Feb;55(2):388–392. doi: 10.1128/iai.55.2.388-392.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Shuford W. W. Survival and growth of Yersinia pestis within macrophages and an effect of the loss of the 47-megadalton plasmid on growth in macrophages. Infect Immun. 1985 Jan;47(1):234–241. doi: 10.1128/iai.47.1.234-241.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikami G. K., Fierer J., Guiney D. G. Plasmid-mediated virulence in Salmonella dublin demonstrated by use of a Tn5-oriT construct. Infect Immun. 1985 Nov;50(2):420–424. doi: 10.1128/iai.50.2.420-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Starnbach M. N., Francis C. L., Stocker B. A., Chatfield S., Dougan G., Falkow S. Identification and characterization of TnphoA mutants of Salmonella that are unable to pass through a polarized MDCK epithelial cell monolayer. Mol Microbiol. 1988 Nov;2(6):757–766. doi: 10.1111/j.1365-2958.1988.tb00087.x. [DOI] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Chiodo V. A. Genetic and DNA sequence analysis of the Salmonella typhimurium virulence plasmid gene encoding the 28,000-molecular-weight protein. Infect Immun. 1990 Aug;58(8):2651–2658. doi: 10.1128/iai.58.8.2651-2658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Cloning and transposon insertion mutagenesis of virulence genes of the 100-kilobase plasmid of Salmonella typhimurium. Infect Immun. 1988 Dec;56(12):3262–3271. doi: 10.1128/iai.56.12.3262-3271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987 Dec;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J., Kotlarski I., Mathan V., Francki K., Rowley D. The colonization of Peyer's patches by a strain of Salmonella typhimurium cured of the cryptic plasmid. J Infect Dis. 1986 Jun;153(6):1119–1125. doi: 10.1093/infdis/153.6.1119. [DOI] [PubMed] [Google Scholar]
- Hackett J., Wyk P., Reeves P., Mathan V. Mediation of serum resistance in Salmonella typhimurium by an 11-kilodalton polypeptide encoded by the cryptic plasmid. J Infect Dis. 1987 Mar;155(3):540–549. doi: 10.1093/infdis/155.3.540. [DOI] [PubMed] [Google Scholar]
- Harris J. R., Wachsmuth I. K., Davis B. R., Cohen M. L. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect Immun. 1982 Sep;37(3):1295–1298. doi: 10.1128/iai.37.3.1295-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan E. J., Fierer J., Chikami G., Guiney D. Natural history of oral Salmonella dublin infection in BALB/c mice: effect of an 80-kilobase-pair plasmid on virulence. J Infect Dis. 1987 Jun;155(6):1254–1259. doi: 10.1093/infdis/155.6.1254. [DOI] [PubMed] [Google Scholar]
- Helmuth R., Stephan R., Bunge C., Hoog B., Steinbeck A., Bulling E. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985 Apr;48(1):175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I. B., Blight M. A., Kenny B. The mechanism of secretion of hemolysin and other polypeptides from gram-negative bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):473–491. doi: 10.1007/BF00763178. [DOI] [PubMed] [Google Scholar]
- Hovi M., Sukupolvi S., Edwards M. F., Rhen M. Plasmid-associated virulence of Salmonella enteritidis. Microb Pathog. 1988 May;4(5):385–391. doi: 10.1016/0882-4010(88)90066-6. [DOI] [PubMed] [Google Scholar]
- Kawahara K., Haraguchi Y., Tsuchimoto M., Terakado N., Danbara H. Evidence of correlation between 50-kilobase plasmid of Salmonella choleraesuis and its virulence. Microb Pathog. 1988 Feb;4(2):155–163. doi: 10.1016/0882-4010(88)90057-5. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Roudier C., Fierer J., Harwood J., Guiney D. Molecular analysis of the virulence locus of the Salmonella dublin plasmid pSDL2. Mol Microbiol. 1991 Feb;5(2):307–316. doi: 10.1111/j.1365-2958.1991.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. A genetic approach to analyzing membrane protein topology. Science. 1986 Sep 26;233(4771):1403–1408. doi: 10.1126/science.3529391. [DOI] [PubMed] [Google Scholar]
- Manoil C., Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels T., Cornelis G. R. Secretion of hybrid proteins by the Yersinia Yop export system. J Bacteriol. 1991 Mar;173(5):1677–1685. doi: 10.1128/jb.173.5.1677-1685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norel F., Coynault C., Miras I., Hermant D., Popoff M. Y. Cloning and expression of plasmid DNA sequences involved in Salmonella serotype typhimurium virulence. Mol Microbiol. 1989 Jun;3(6):733–743. doi: 10.1111/j.1365-2958.1989.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pepe J. C., Miller V. L. The Yersinia enterocolitica inv gene product is an outer membrane protein that shares epitopes with Yersinia pseudotuberculosis invasin. J Bacteriol. 1990 Jul;172(7):3780–3789. doi: 10.1128/jb.172.7.3780-3789.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu Rev Genet. 1990;24:67–90. doi: 10.1146/annurev.ge.24.120190.000435. [DOI] [PubMed] [Google Scholar]
- Roudier C., Krause M., Fierer J., Guiney D. G. Correlation between the presence of sequences homologous to the vir region of Salmonella dublin plasmid pSDL2 and the virulence of twenty-two Salmonella serotypes in mice. Infect Immun. 1990 May;58(5):1180–1185. doi: 10.1128/iai.58.5.1180-1185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigeti J. S., Guiney D. G., Jr, Davis C. E. Mechanism of action of metronidazole on Bacteroides fragilis. J Infect Dis. 1983 Dec;148(6):1083–1089. doi: 10.1093/infdis/148.6.1083. [DOI] [PubMed] [Google Scholar]
- Small P. L., Falkow S. Identification of regions on a 230-kilobase plasmid from enteroinvasive Escherichia coli that are required for entry into HEp-2 cells. Infect Immun. 1988 Jan;56(1):225–229. doi: 10.1128/iai.56.1.225-229.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Cibull M. L. Differential clearance and host-pathogen interactions of YopE- and YopK- YopL- Yersinia pestis in BALB/c mice. Infect Immun. 1989 Apr;57(4):1200–1210. doi: 10.1128/iai.57.4.1200-1210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch J. L., Rabert D. K., Jones G. W. Plasmid-associated resistance of Salmonella typhimurium to complement activated by the classical pathway. Infect Immun. 1987 Nov;55(11):2645–2652. doi: 10.1128/iai.55.11.2645-2652.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989 Dec;3(12):1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Williamson C. M., Baird G. D., Manning E. J. A common virulence region on plasmids from eleven serotypes of Salmonella. J Gen Microbiol. 1988 Apr;134(4):975–982. doi: 10.1099/00221287-134-4-975. [DOI] [PubMed] [Google Scholar]
- Yakobson E. A., Guiney D. G., Jr Conjugal transfer of bacterial chromosomes mediated by the RK2 plasmid transfer origin cloned into transposon Tn5. J Bacteriol. 1984 Oct;160(1):451–453. doi: 10.1128/jb.160.1.451-453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]





