Abstract
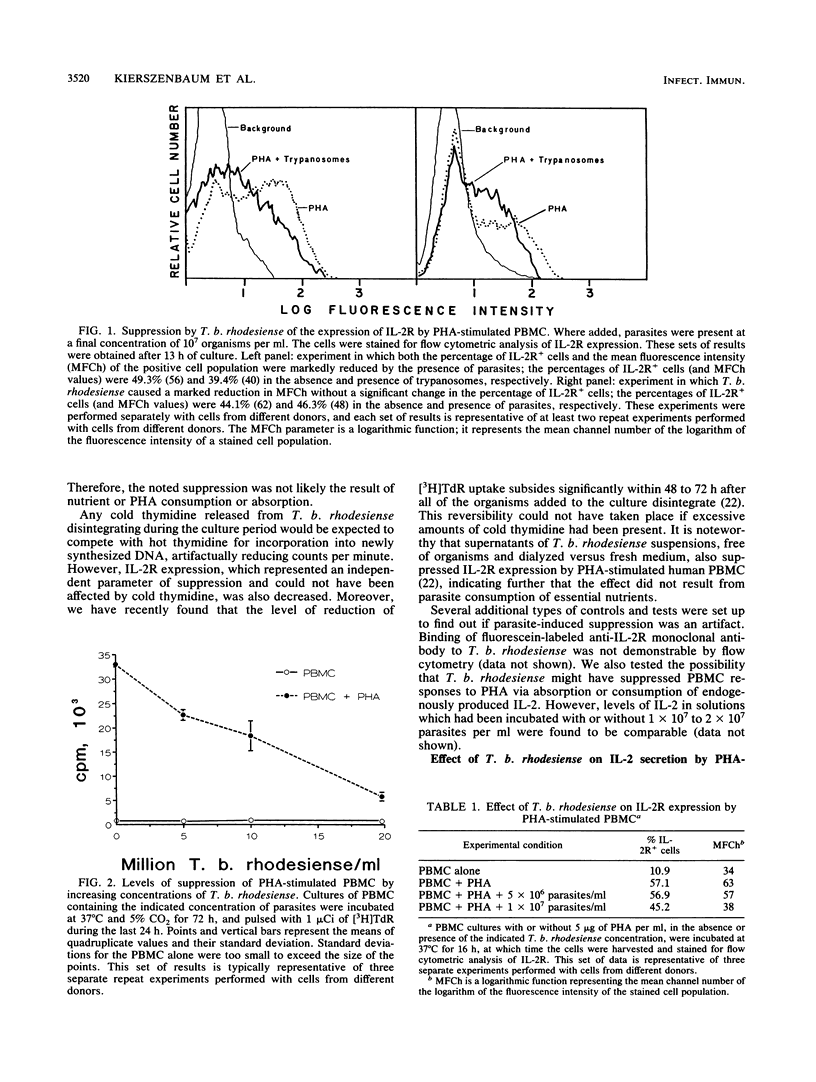
We studied the suppressive effects induced in phytohemagglutinin (PHA)-stimulated human peripheral blood mononuclear cells (PBMC) by purified blood forms of Trypanosoma brucei rhodesiense. The parasite was found to markedly impair lymphocyte proliferation (measured in terms of [3H]thymidine incorporation). The extent of this effect increased with parasite concentration and was not due to mitogen absorption, depletion of medium nutrients, or PBMC killing by the parasite. Significant reductions in interleukin-2 receptor (IL-2R) expression, determined by flow cytometric analysis, were also observed in PHA-stimulated PBMC cultured in the presence of T. b. rhodesiense as evidenced by marked decreases in the surface density of the receptor. Concomitant decreases in the percentage of IL-2R+ cells were recorded in approximately half of the experiments. A discrete, dimly stained subpopulation of IL-2R+ cells were consistently demonstrable whether or not a reduction in the percentage of IL-2R+ cells occurred. Living, but not glutaraldehyde-fixed, parasites suppressed IL-2R expression. In kinetic studies, a low but reproducible level of suppression of IL-2R was demonstrable as early as 6 h after PHA stimulation; the extent of this effect became considerably more pronounced as additional culture time elapsed. Levels of IL-2 biological activity in cocultures of T. b. rhodesiense with PHA-stimulated PBMC were comparable with or higher than those present in control cultures lacking the parasite. Therefore, insufficient levels of this cytokine would be an unlikely explanation for the noted suppression of IL-2R expression and lymphoproliferation. These effects of T. b. rhodesiense could represent an important component of the mechanism by which immunosuppression develops in African sleeping sickness.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakhiet M., Olsson T., van der Meide P., Kristensson K. Depletion of CD8+ T cells suppresses growth of Trypanosoma brucei brucei and interferon-gamma) production in infected rats. Clin Exp Immunol. 1990 Aug;81(2):195–199. doi: 10.1111/j.1365-2249.1990.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Sutton C. J., Morris A. G., Askonas B. A. Production of interferons during experimental African trypanosomiasis. Clin Exp Immunol. 1983 Apr;52(1):135–143. [PMC free article] [PubMed] [Google Scholar]
- Beltz L. A., Kierszenbaum F., Sztein M. B. Selective suppressive effects of Trypanosoma cruzi on activated human lymphocytes. Infect Immun. 1989 Aug;57(8):2301–2305. doi: 10.1128/iai.57.8.2301-2305.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz L. A., Sztein M. B., Kierszenbaum F. Novel mechanism for Trypanosoma cruzi-induced suppression of human lymphocytes. Inhibition of IL-2 receptor expression. J Immunol. 1988 Jul 1;141(1):289–294. [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choromanski L., Kuhn R. E. Interleukin 2 enhances specific and nonspecific immune responses in experimental Chagas' disease. Infect Immun. 1985 Nov;50(2):354–357. doi: 10.1128/iai.50.2.354-357.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini A. C., Clayton C., Askonas B. A., Ogilvie B. M. Suppressor cells and loss of B-cell potential in mice infected with Trypanosoma brucei. Clin Exp Immunol. 1977 Jul;29(1):122–131. [PMC free article] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Cuna W. R., Beltz L. A., Sztein M. B. Trypanosoma cruzi reduces the number of high-affinity IL-2 receptors on activated human lymphocytes by suppressing the expression of the p55 and p70 receptor components. J Immunol. 1989 Jul 1;143(1):275–279. [PubMed] [Google Scholar]
- Kierszenbaum F., Sztein M. B., Beltz L. A. Decreased human IL-2 receptor expression due to a protozoan pathogen. Immunol Today. 1989 Apr;10(4):129–131. doi: 10.1016/0167-5699(89)90246-6. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Leichtling K. D., Serrate S. A., Sztein M. B. Thymosin alpha 1 modulates the expression of high affinity interleukin-2 receptors on normal human lymphocytes. Int J Immunopharmacol. 1990;12(1):19–29. doi: 10.1016/0192-0561(90)90064-t. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Ashwell J. D. Interleukin 2 upregulates expression of its receptor on a T cell clone. J Exp Med. 1985 Jun 1;161(6):1575–1580. doi: 10.1084/jem.161.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. A., Pearson T. W., Gauldie J. Interleukin-1 and interleukin-2 production in resistant and susceptible inbred mice infected with Trypanosoma congolense. Immunology. 1986 Feb;57(2):291–296. [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H., Urdal D. L., Kilian P. L., Farrar J. J. Induction and upregulation by interleukin 2 of high-affinity interleukin 2 receptors on thymocytes and T cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8663–8666. doi: 10.1073/pnas.82.24.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileghem M., Darji A., Remels L., Hamers R., De Baetselier P. Different mechanisms account for the suppression of interleukin 2 production and the suppression of interleukin 2 receptor expression in Trypanosoma brucei-infected mice. Eur J Immunol. 1989 Jan;19(1):119–124. doi: 10.1002/eji.1830190119. [DOI] [PubMed] [Google Scholar]
- Sileghem M., Hamers R., De Baetselier P. Experimental Trypanosoma brucei infections selectively suppress both interleukin 2 production and interleukin 2 receptor expression. Eur J Immunol. 1987 Oct;17(10):1417–1421. doi: 10.1002/eji.1830171005. [DOI] [PubMed] [Google Scholar]
- Sizemore R. C., Mansfield J. M. Lymphocyte function in experimental African trypanosomiasis. VII. Loss of antigen-nonspecific suppressor-T-cell activity. Cell Immunol. 1984 Sep;87(2):684–691. doi: 10.1016/0008-8749(84)90036-4. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein M. B., Kierszenbaum F. A soluble factor from Trypanosoma brucei rhodesiense that prevents progression of activated human T lymphocytes through the cell cycle. Immunology. 1991 Jun;73(2):180–185. [PMC free article] [PubMed] [Google Scholar]


