Abstract
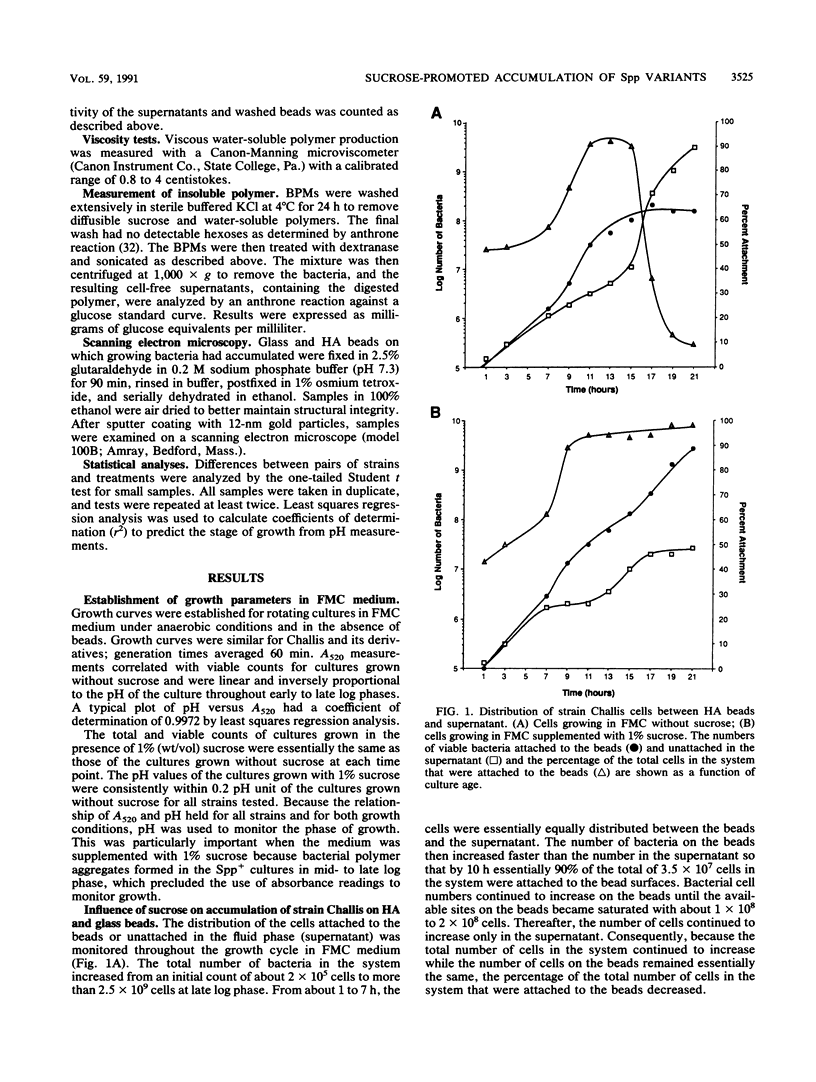
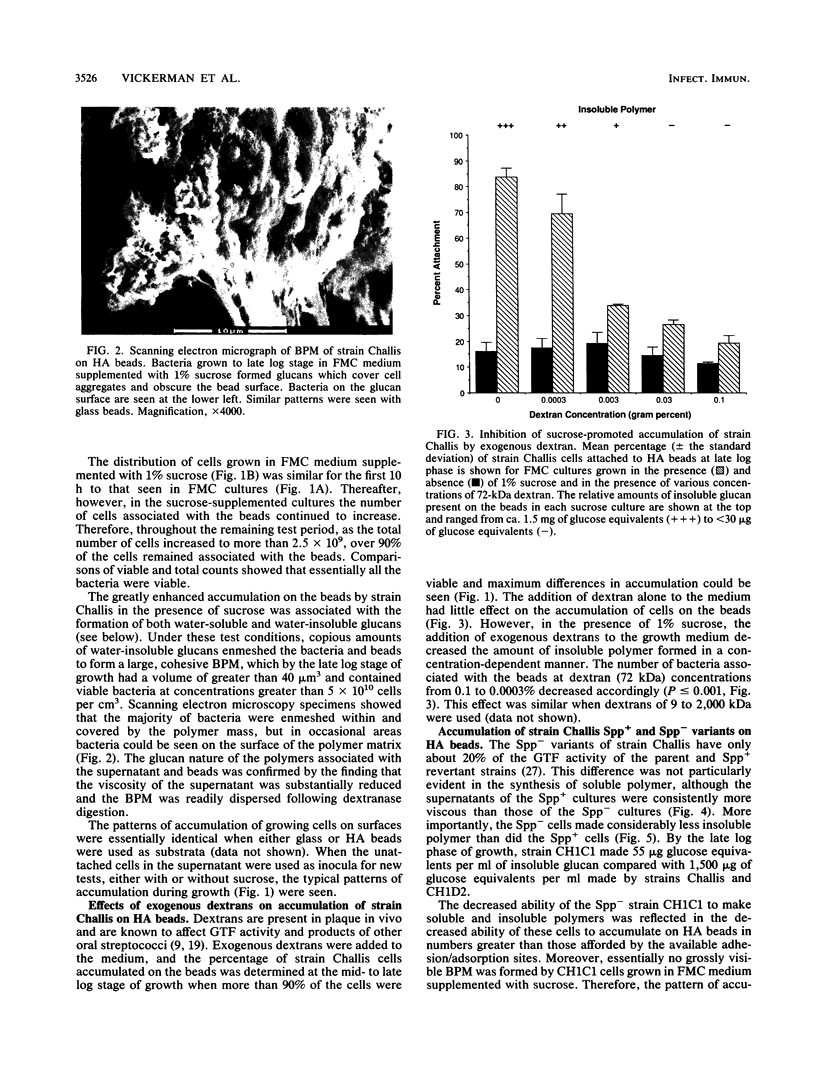
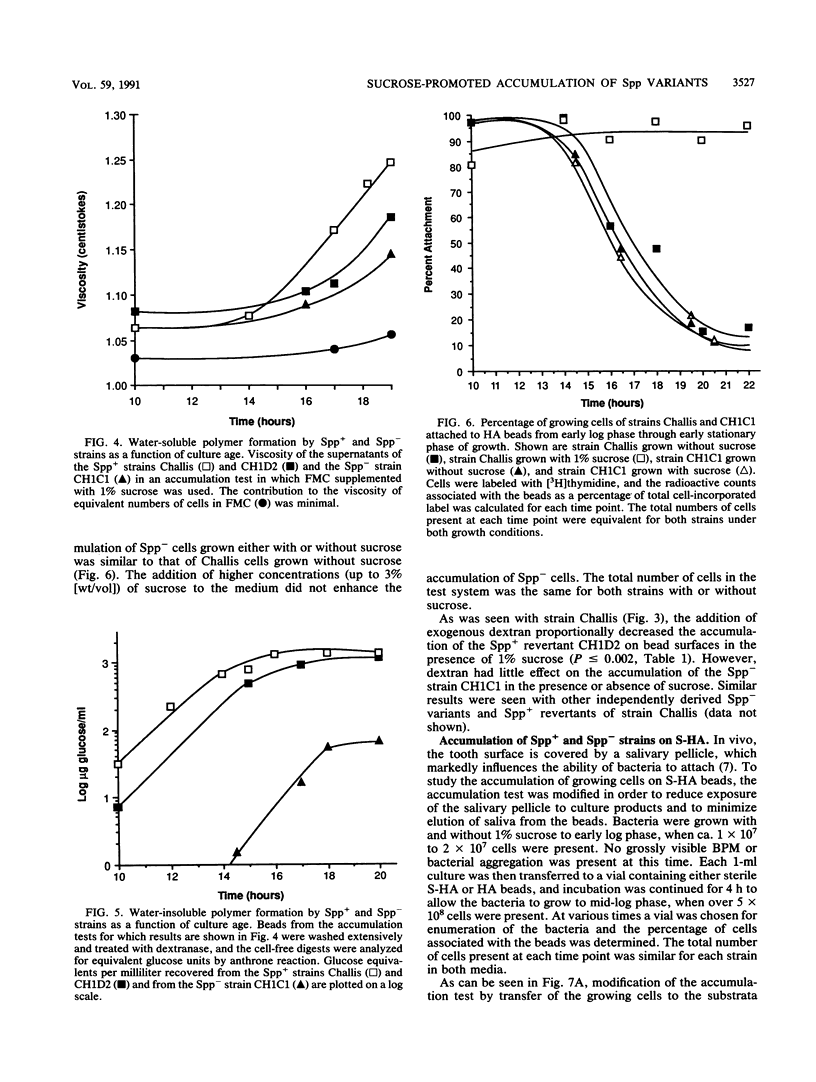
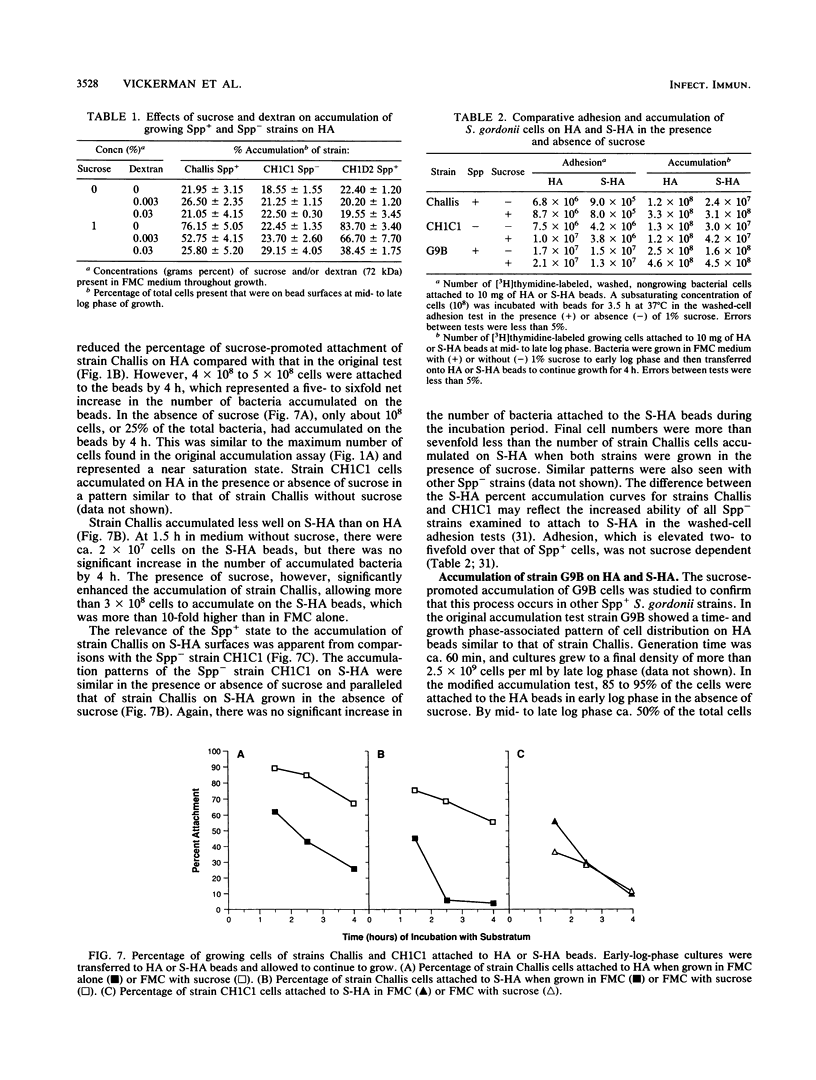
Streptococcus gordonii exhibits a phase variation involving expression of high (Spp+) or low (Spp-) glucosyltransferase activity. The related bacterial accumulation on hydroxyapatite (HA) and saliva-coated HA surfaces was examined and found to be significant. Spp+ cells growing anaerobically in a defined medium utilize about 30% of the glucose available from sucrose to make insoluble glucans. These glucans formed cohesive masses on HA beads, which contained 80 to 90% of the total bacteria. The bacterial polymer mass had a volume of about 40 microns3 and contained more than 5 x 10(10) viable cells per cm3. In the absence of sucrose, the beads were saturated by 1 x 10(8) to 2 x 10(8) Spp+ cells. Spp- bacteria, which make 30-fold less glucan than do Spp+ bacteria, did not accumulate on surfaces in numbers significantly above the saturation level of 1 x 10(8) to 2 x 10(8) cells in the presence or absence of sucrose. Insoluble glucan synthesized by Spp+ cells from sucrose also enabled these bacteria to accumulate on saliva-coated HA seven times more effectively than the Spp- cells and 10 times more effectively than the Spp+ cells grown in medium without sucrose.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Golub E., Holt S. C., Rosan B. In vitro studies of dental plaque formation: adsorption of oral streptococci to hydroxyaptite. Infect Immun. 1979 Aug;25(2):717–728. doi: 10.1128/iai.25.2.717-728.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan R. A., Jenkinson H. F. Glucosyltransferase production by Streptococcus sanguis Challis and comparison with other oral streptococci. Oral Microbiol Immunol. 1990 Apr;5(2):63–71. doi: 10.1111/j.1399-302x.1990.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M., Parrish K., Kessler R. E., Pyle C., Jr, Taylor K. G., Ciardi J. E., Doyle R. J. Glucan-binding factor in saliva. Infect Immun. 1988 Nov;56(11):2912–2917. doi: 10.1128/iai.56.11.2912-2917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Grahame D. A., Mayer R. M. The origin and composition of multiple forms of dextransucrase from Streptococcus sanguis. Biochim Biophys Acta. 1984 Apr 27;786(1-2):42–48. doi: 10.1016/0167-4838(84)90151-1. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson M. C., Curtiss R., 3rd Regulation of expression of Streptococcus mutans genes important to virulence. Infect Immun. 1990 Feb;58(2):464–470. doi: 10.1128/iai.58.2.464-470.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Mayer R. M. Dextransucrase: a glucosyltransferase from Streptococcus sanguis. Methods Enzymol. 1987;138:649–661. doi: 10.1016/0076-6879(87)38059-0. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Kiziuta Z., White J. C. Synthesis of a Polysaccharide from Sucrose by Streptococcus S.B.E. J Bacteriol. 1946 Jun;51(6):711–716. doi: 10.1128/jb.51.6.711-716.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24(4):267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Use of triton X-100 to overcome the inhibition of fructosyltransferase by SDS. Anal Biochem. 1979 Aug;97(1):173–175. doi: 10.1016/0003-2697(79)90342-7. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Harlander S. K., Bracke J. W., Ostrum L. C., Maltais J. A., Billings R. J. Streptococcus mutans dextransucrase: stimulation by phospholipids from human sera and oral fluids. Infect Immun. 1978 Dec;22(3):714–720. doi: 10.1128/iai.22.3.714-720.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G., Sulavik M. C., Jones G. W., Clewell D. B. Spontaneous switching of the sucrose-promoted colony phenotype in Streptococcus sanguis. Infect Immun. 1989 Dec;57(12):3945–3948. doi: 10.1128/iai.57.12.3945-3948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEMM E. W., WILLIS A. J. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954 Jul;57(3):508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Zehnder A. J. Influence of interfaces on microbial activity. Microbiol Rev. 1990 Mar;54(1):75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



