Abstract
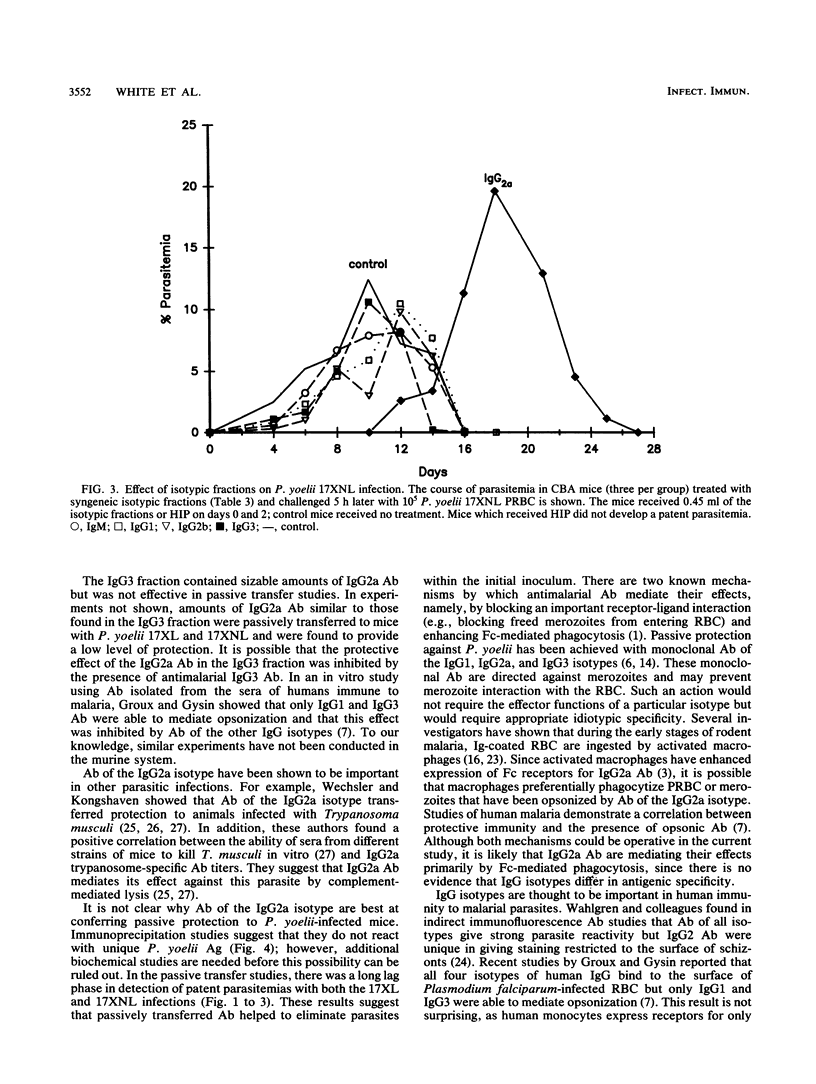
Previous studies have demonstrated the importance of antibodies in mediating immunity to malaria, but the relative contribution of the different immunoglobulin isotypes has not been assessed. In this study, hyperimmune plasma was generated against Plasmodium yoelii and separated by protein A-Sepharose chromatography into fractions containing immunoglobulin G1 (IgG1), IgG2a, IgG2b, or IgG3 antibodies and the remaining nonbinding plasma proteins, including IgM. Following concentration, the antimalarial titer of each isotypic fraction was approximately equivalent to the corresponding isotype in hyperimmune plasma. The isotypic fractions were passively transferred to BALB/c and outbred ICR mice prior to challenge with virulent P. yoelii 17XL and to CBA/CaJ mice challenged with avirulent P. yoelii 17XNL. Only mice receiving IgG2a antibodies experienced an altered course of infection. Immunoprecipitation studies showed that all four IgG isotypes appear to recognize a similar set of antigens. These results suggest that antimalarial antibodies of the IgG2a isotype play a dominant role in modulating P. yoelii parasitemias.
Full text
PDF

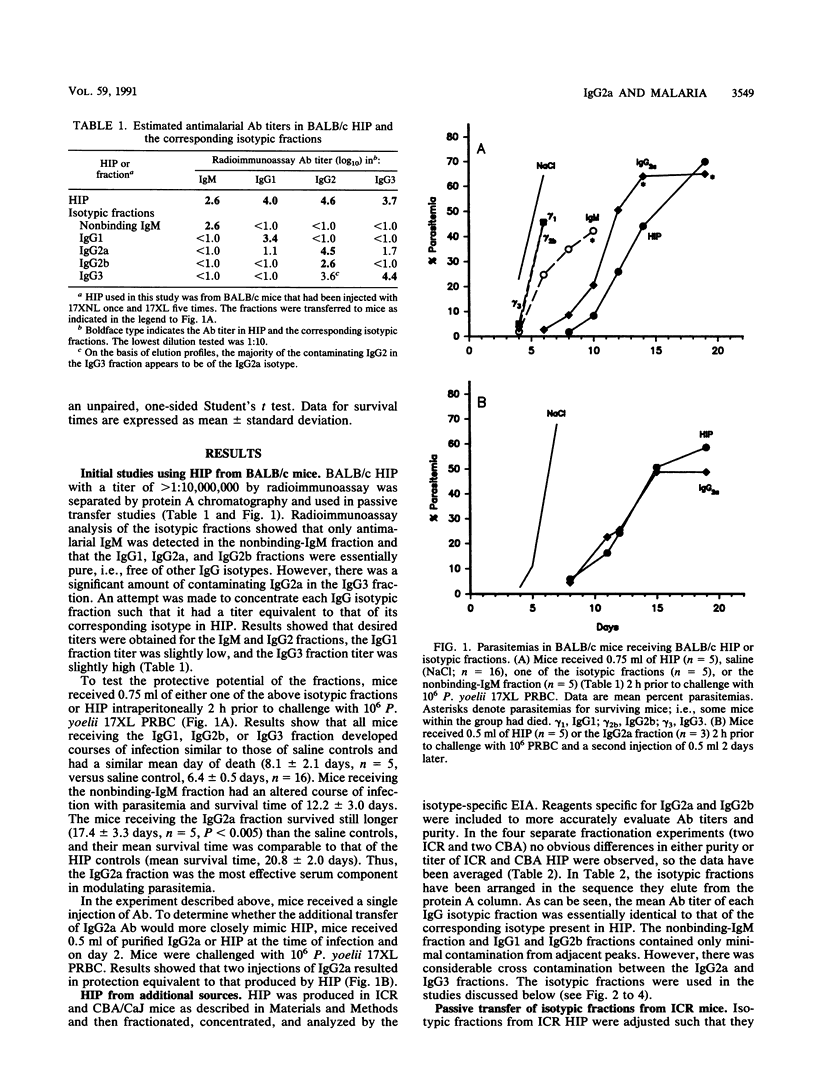


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks C., Kreier J. P. Role of the surface coat in in vitro attachment and phagocytosis of Plasmodium berghei by peritoneal macrophages. Infect Immun. 1978 Jun;20(3):827–835. doi: 10.1128/iai.20.3.827-835.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Bampton M., Gordon S. Macrophage activation selectively enhances expression of Fc receptors for IgG2a. J Exp Med. 1983 Feb 1;157(2):807–812. doi: 10.1084/jem.157.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facer C. A. Direct antiglobulin reactions in Gambian children with P. falciparum malaria. III. Expression of IgG subclass determinants and genetic markers and association with anaemia. Clin Exp Immunol. 1980 Jul;41(1):81–90. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Parish C. R. Plasmodium yoelii: antibody and the maintenance of immunity in BALB/c mice. Exp Parasitol. 1981 Aug;52(1):18–24. doi: 10.1016/0014-4894(81)90056-4. [DOI] [PubMed] [Google Scholar]
- Freeman R. R., Trejdosiewicz A. J., Cross G. A. Protective monoclonal antibodies recognising stage-specific merozoite antigens of a rodent malaria parasite. Nature. 1980 Mar 27;284(5754):366–368. doi: 10.1038/284366a0. [DOI] [PubMed] [Google Scholar]
- Groux H., Gysin J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: functional role of IgG subclasses. Res Immunol. 1990 Jul-Aug;141(6):529–542. doi: 10.1016/0923-2494(90)90021-p. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Smith P. M., Mitchell G. F. Removal of leucocytes from red cells in Plasmodium berghei-infected mouse blood and purification of schizont-infected cells. Ann Trop Med Parasitol. 1978 Dec;72(6):573–575. doi: 10.1080/00034983.1978.11719363. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. II. The passive transfer of immunity with serum and cells. Immunology. 1978 Jan;34(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Kenney J. S., Hughes B. W., Masada M. P., Allison A. C. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J Immunol Methods. 1989 Jul 26;121(2):157–166. doi: 10.1016/0022-1759(89)90156-7. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Taylor D. W., Evans C. B., Asofsky R. Radioimmunoassay for detecting antibodies against murine malarial parasite antigens: monoclonal antibodies recognizing Plasmodium yoelii antigens. J Immunol. 1980 Dec;125(6):2565–2569. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Evans C. B., Asofsky R., Taylor D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 1984 Sep;87(2):452–461. doi: 10.1016/0008-8749(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Seppälä I., Sarvas H., Péterfy F., Mäkelä O. The four subclasses of IgG can be isolated from mouse serum by using Protein A-Sepharose. Scand J Immunol. 1981 Oct;14(4):335–342. doi: 10.1111/j.1365-3083.1981.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Nussenzweig R. S., Bianco C. Immune phagocytosis in murine malaria. J Exp Med. 1979 Jun 1;149(6):1288–1298. doi: 10.1084/jem.149.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Munoz P. A., Kim K. J., Evans C. B., Asofsky R. Plasmodium yoelii: comparison of indirect immunofluorescence and radioimmunoassay for detecting monoclonal antibodies to malaria. Exp Parasitol. 1982 Jun;53(3):362–370. doi: 10.1016/0014-4894(82)90079-0. [DOI] [PubMed] [Google Scholar]
- Topley E., Bruce-Chwatt L. J., Dorrell J. Haematological study of a rodent malaria model. Trans R Soc Trop Med Hyg. 1970;64(1):7–8. [PubMed] [Google Scholar]
- Tosta C. E., Wedderburn N. Immune phagocytosis of Plasmodium yoelii-infected erythrocytes by macrophages and eosinophils. Clin Exp Immunol. 1980 Oct;42(1):114–120. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Characterization of antibodies mediating protection and cure of Trypanosoma musculi infection in mice. Infect Immun. 1985 Jun;48(3):787–794. doi: 10.1128/iai.48.3.787-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Further characterization of the curative antibodies in Trypanosoma musculi infection. Infect Immun. 1988 Sep;56(9):2379–2384. doi: 10.1128/iai.56.9.2379-2384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Heat-labile IgG2a antibodies affect cure of Trypanosoma musculi infection in C57BL/6 mice. J Immunol. 1986 Nov 1;137(9):2968–2972. [PubMed] [Google Scholar]