Abstract
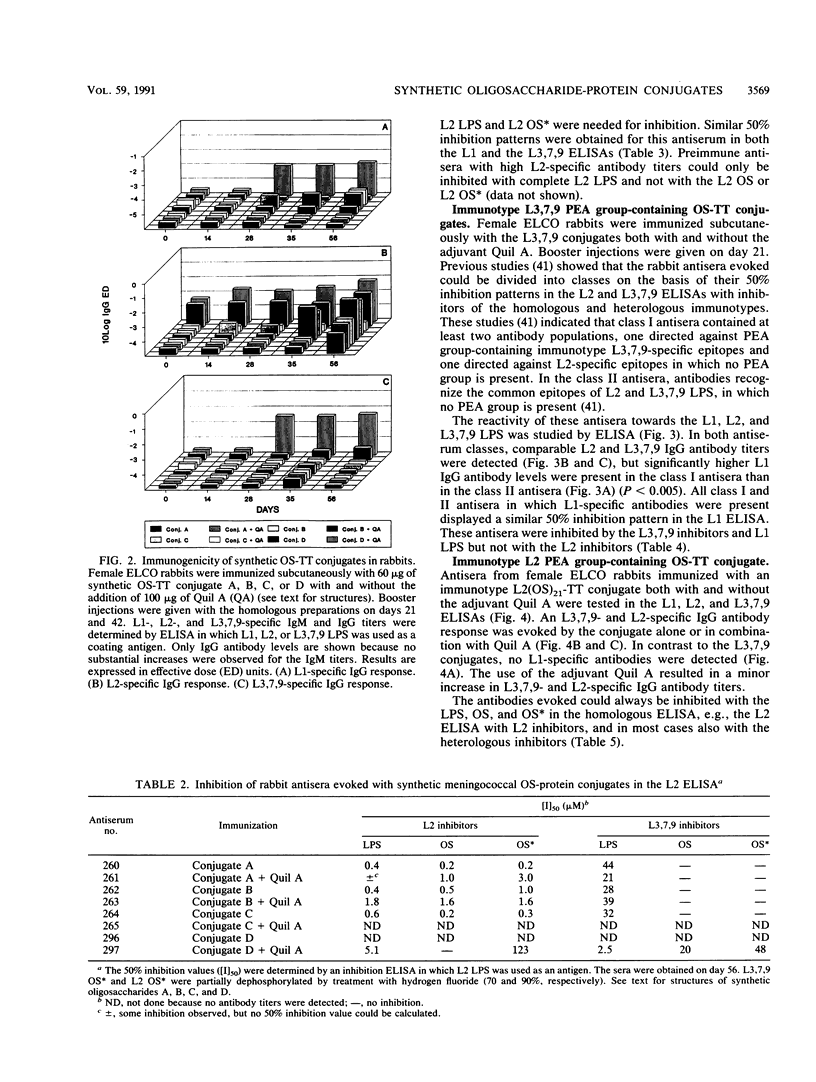
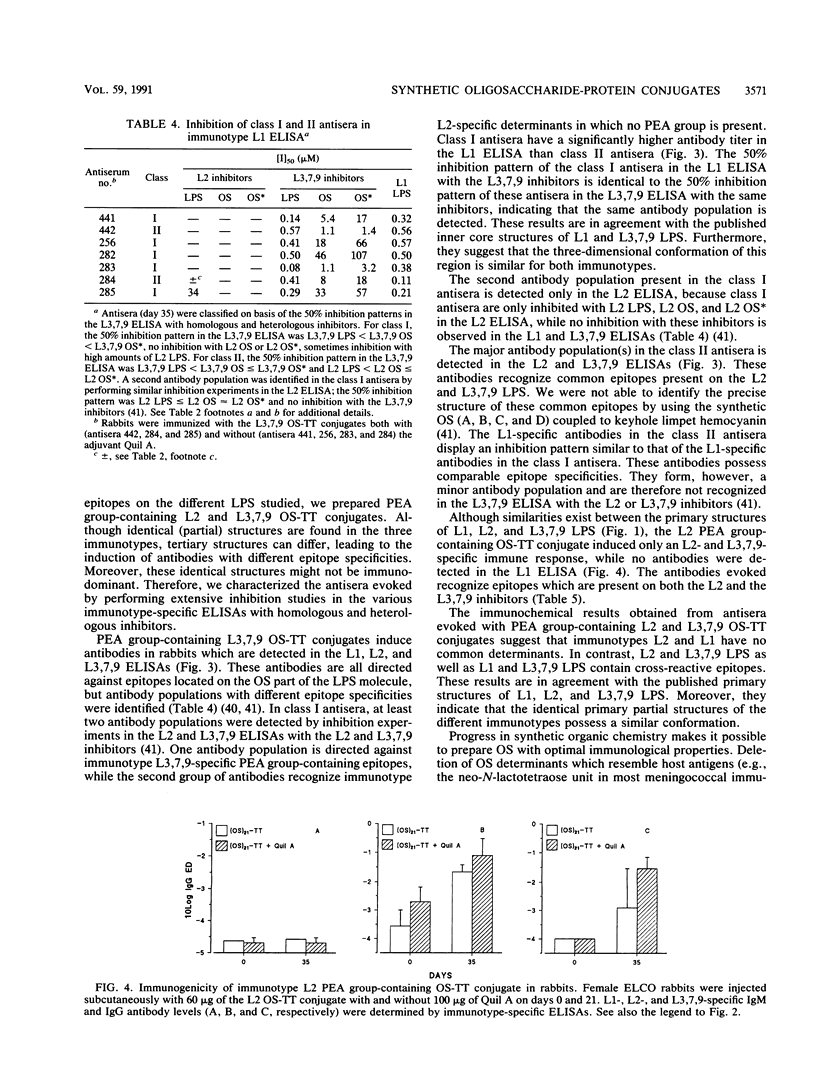
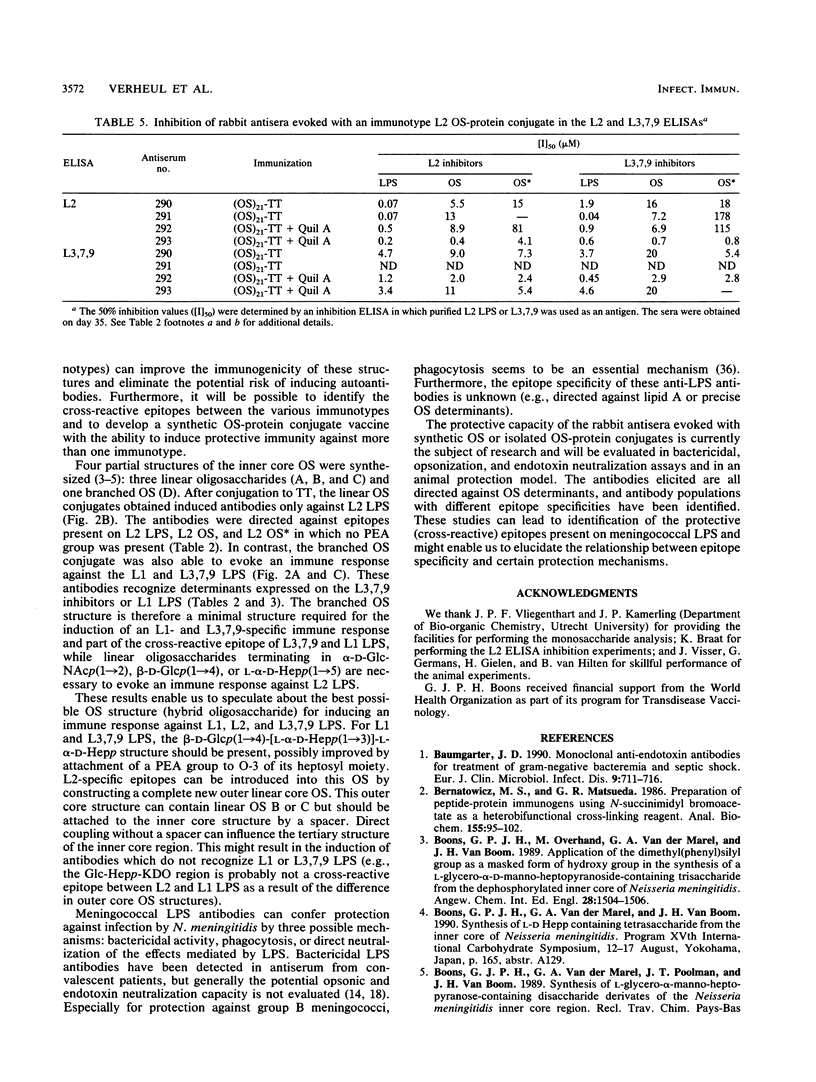
The 12 types of meningococcal lipopolysaccharide (LPS) (immunotypes) contain immunotype-specific and cross-reactive epitopes situated on the oligosaccharide part of the LPS molecules. To identify useful cross-reactive epitopes and to determine minimal oligosaccharide structures required for the induction of an immune response against the most prevalent immunotypes, L1, L2, and L3,7,9, synthetic as well as native LPS-derived oligosaccharides were conjugated with tetanus toxoid. L3,7,9 phosphoethanolamine (PEA) group-containing oligosaccharide-tetanus toxoid conjugates evoked high immunoglobulin G (IgG) antibody levels in rabbits which were detected by an L2-, L3,7,9-, and, depending on the antiserum, L1-specific enzyme-linked immunosorbent assay (ELISA). Inhibition studies revealed that an identical antibody population was detected by L1 and L3,7,9 ELISA, indicating a similar tertiary structure of the inner core oligosaccharide of these two immunotypes. These antibodies recognize PEA group-containing epitopes present on the L1 and L3,7,9 LPS. An L2 PEA group-containing oligosaccharide-tetanus toxoid conjugate elicited L2- and L3,7,9-specific IgG antibodies, but in contrast with the L3,7,9 conjugates, no L1-specific IgG antibodies were evoked. These results indicate that L1 and L2 LPS do not contain cross-reactive epitopes, whereas both L2 and L3,7,9 LPS and L1 and L3,7,9 LPS possess common determinants. Three linear oligosaccharides and one branched oligosaccharide, representing partial structures of the inner core oligosacchardes of meningococcal LPS, were synthesized. Only the branched synthetic oligosaccharide-containing conjugate was able to induce and L1- and L3,7,9-specific immune response, whereas the linear oligosaccharide-protein conjugates evoked L2-specific immune responses. The branched oligosaccharide (beta-D-Glcp(1----4)-[L-alpha-D-Hepp(1----3)]-L-alpha-D-Hepp ) is therefore considered a minimal structure required for the induction of an immune response against L1 and L3,7,9 LPS and part of a cross-reactive epitope between these two immunotypes. For L2-specific immune responses, oligosaccharide structures terminating in beta-D-Glcp(1----4), alpha-D-GlcNAcp(1----2), or L-alpha-D-Hepp(1----5) are needed. The results suggest that it is possible to prepare an oligosaccharide structure with the ability to evoke an immune response against L1, L2, and L3,7,9 LPS. A feasible structure for such a "hybrid" oligosaccharide is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner J. D. Monoclonal anti-endotoxin antibodies for the treatment of gram-negative bacteremia and septic shock. Eur J Clin Microbiol Infect Dis. 1990 Oct;9(10):711–716. doi: 10.1007/BF02184682. [DOI] [PubMed] [Google Scholar]
- Bernatowicz M. S., Matsueda G. R. Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent. Anal Biochem. 1986 May 15;155(1):95–102. doi: 10.1016/0003-2697(86)90231-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Kierulf P., Gaustad P., Skulberg A., Bruun J. N., Halvorsen S., Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989 Feb;159(2):195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Mollnes T. E., Kierulf P. Complement activation and endotoxin levels in systemic meningococcal disease. J Infect Dis. 1989 Jul;160(1):58–65. doi: 10.1093/infdis/160.1.58. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretsen L. F., Kierulf P., Brandtzaeg P. Extreme plasminogen activator inhibitor and endotoxin values in patients with meningococcal disease. Thromb Res. 1986 Jun 1;42(5):713–716. doi: 10.1016/0049-3848(86)90351-8. [DOI] [PubMed] [Google Scholar]
- Frasch C. E. Role of protein serotype antigens in protection against disease due to Neisseria meningitidis. J Infect Dis. 1977 Aug;136 (Suppl):S84–S90. doi: 10.1093/infdis/136.supplement.s84. [DOI] [PubMed] [Google Scholar]
- Gibson B. W., Webb J. W., Yamasaki R., Fisher S. J., Burlingame A. L., Mandrell R. E., Schneider H., Griffiss J. M. Structure and heterogeneity of the oligosaccharides from the lipopolysaccharides of a pyocin-resistant Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1989 Jan;86(1):17–21. doi: 10.1073/pnas.86.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Apicella M. A., Greenwood B., Mäkelä P. H. Vaccines against encapsulated bacteria: a global agenda. Rev Infect Dis. 1987 Jan-Feb;9(1):176–188. doi: 10.1093/clinids/9.1.176. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L., Broud D. D., Goroff D. K., Baker C. J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984 Jul;150(1):71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Beurret M., Gamian A., Michon F. Structure and immunochemistry of meningococcal lipopolysaccharides. Antonie Van Leeuwenhoek. 1987;53(6):519–522. doi: 10.1007/BF00415511. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Ashton F. E. Conjugation of meningococcal lipopolysaccharide R-type oligosaccharides to tetanus toxoid as route to a potential vaccine against group B Neisseria meningitidis. Infect Immun. 1984 Jan;43(1):407–412. doi: 10.1128/iai.43.1.407-412.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide-tetanus toxoid conjugate vaccine. J Immunol. 1986 Sep 1;137(5):1708–1713. [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp H. A., Morgan M. R. Studies on the detrimental effects of bivalent binding in a microtitration plate ELISA and possible remedies. J Immunol Methods. 1986 Nov 20;94(1-2):65–72. doi: 10.1016/0022-1759(86)90216-4. [DOI] [PubMed] [Google Scholar]
- Leinikki P. O., Shekarchi I., Dorsett P., Sever J. L. Enzyme-linked immunosorbent assay determination of specific rubella antibody levels in micrograms of immunoglobulin G per milliliter of serum in clinical samples. J Clin Microbiol. 1978 Oct;8(4):419–423. doi: 10.1128/jcm.8.4.419-423.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandine E., Salles M. F., Zalisz R., Guenounou M., Smets P. Murine monoclonal antibodies to Klebsiella pneumoniae protect against lethal endotoxemia and experimental infection with capsulated K. pneumoniae. Infect Immun. 1990 Sep;58(9):2828–2833. doi: 10.1128/iai.58.9.2828-2833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F., Beurret M., Gamian A., Brisson J. R., Jennings H. J. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem. 1990 May 5;265(13):7243–7247. [PubMed] [Google Scholar]
- Morein B. Immunopotentiating complexes. Acta Trop Suppl. 1987 Jun;12:98–103. [PubMed] [Google Scholar]
- Morrison D. C. Bacterial endotoxins and pathogenesis. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S733–S747. doi: 10.1093/clinids/5.supplement_4.s733. [DOI] [PubMed] [Google Scholar]
- Richter A. W., Eby R. Studies on artificial oligosaccharide-protein antigens: induction of precipitating antibodies to defined epitopes on natural and synthetic dextrans and mannans. Mol Immunol. 1985 Jan;22(1):29–36. doi: 10.1016/0161-5890(85)90031-8. [DOI] [PubMed] [Google Scholar]
- Ross S. C., Rosenthal P. J., Berberich H. M., Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987 Jun;155(6):1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- Rozalski A., Brade L., Kuhn H. M., Brade H., Kosma P., Appelmelk B. J., Kusumoto S., Paulsen H. Determination of the epitope specificity of monoclonal antibodies against the inner core region of bacterial lipopolysaccharides by use of 3-deoxy-D-manno-octulosonate-containing synthetic antigens. Carbohydr Res. 1989 Oct 31;193:257–270. doi: 10.1016/0008-6215(89)85124-9. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Leinonen M., Abdillahi H., Poolman J. T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989 Aug;7(4):325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Appelmelk B. J., Buurman W. A., Bayston K. F., Cohen J. Monoclonal antibody to endotoxin core protects mice from Escherichia coli sepsis by a mechanism independent of tumor necrosis factor and interleukin-6. J Infect Dis. 1990 Aug;162(2):454–459. doi: 10.1093/infdis/162.2.454. [DOI] [PubMed] [Google Scholar]
- Verheul A. F., Braat A. K., Leenhouts J. M., Hoogerhout P., Poolman J. T., Snippe H., Verhoef J. Preparation, characterization, and immunogenicity of meningococcal immunotype L2 and L3,7,9 phosphoethanolamine group-containing oligosaccharide-protein conjugates. Infect Immun. 1991 Mar;59(3):843–851. doi: 10.1128/iai.59.3.843-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- van Dam G. J., Verheul A. F., Zigterman G. J., de Reuver M. J., Snippe H. Estimation of the avidity of antibodies in polyclonal antisera against Streptococcus pneumoniae type 3 by inhibition ELISA. Mol Immunol. 1989 Mar;26(3):269–274. doi: 10.1016/0161-5890(89)90080-1. [DOI] [PubMed] [Google Scholar]


