Abstract
The diagnosis of viral infections is important for the accurate management of patients with infectious diseases and for the monitoring of the course of epidemics in susceptible populations. The utility of traditional viral diagnostic assays is limited by the time, expense, and expertise required for the performance of tissue culture techniques. Similarly, the application of immunoassay techniques has been inhibited by the limited degrees of sensitivity and specificity which can be attained by most immunoassay methods. Recently, techniques for the identification of DNA and RNA have been applied to the detection of viral nucleic acids in clinical samples. Such assays have a number of potential advantages over corresponding immunoassays directed at the detection of viral antigens. In order to be generally applicable to clinical diagnosis, however, formats for the detection of viral nucleic acids have to be devised which allow for the reproducible quantitation of target DNA or RNA in human body fluids. Furthermore, formats need to be devised which allow enhanced assay sensitivity while maintaining high degrees of specificity and reproducibility. The use of non-isotopic labeling, liquid-phase hybridization, and target amplification techniques offers partial solutions to these problems. The development of practical assays for the detection of viral nucleic acids under a broad range of clinical and laboratory conditions would represent a major advance in the ability of physicians to care for patients with suspected infections.
Full text
PDF


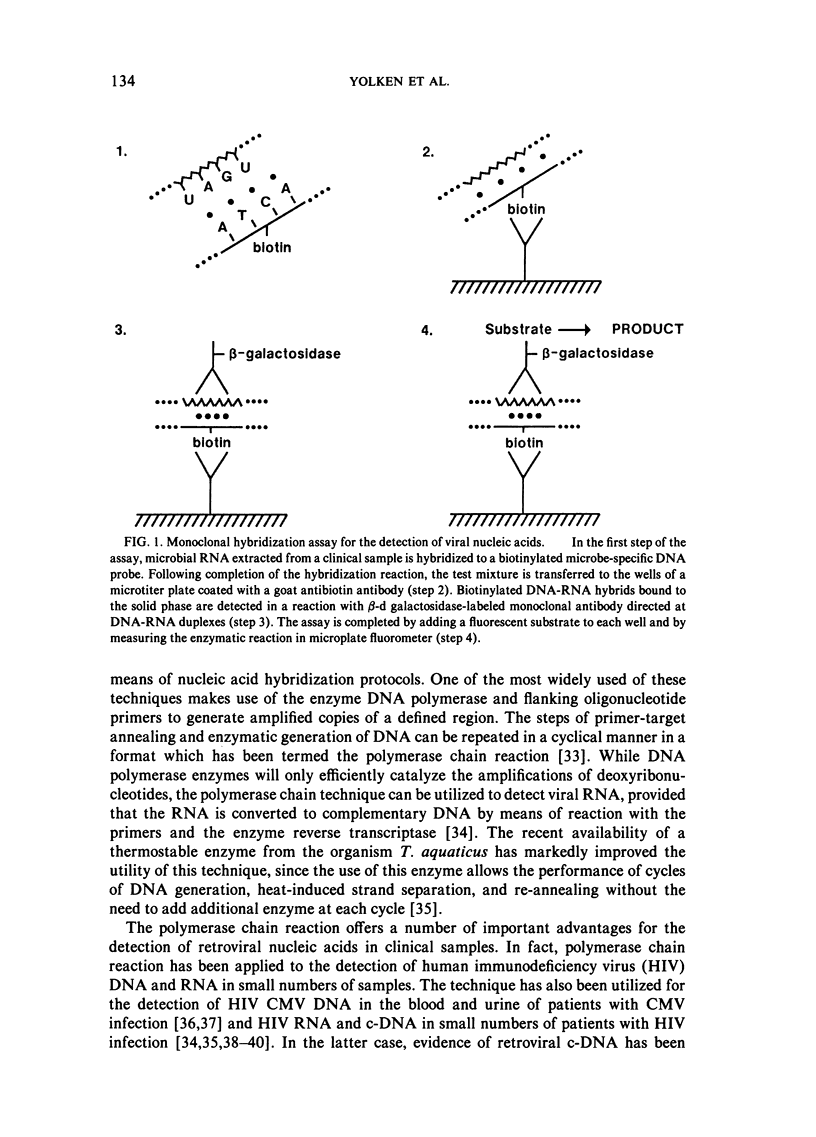
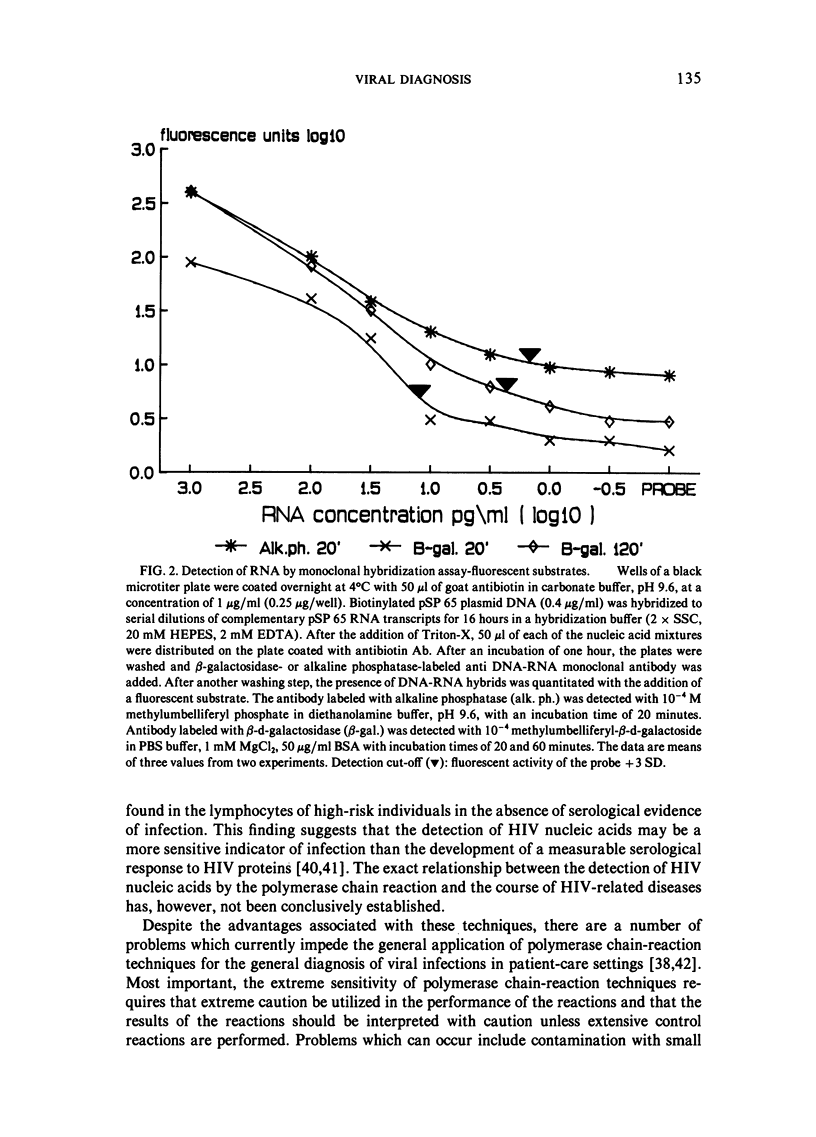
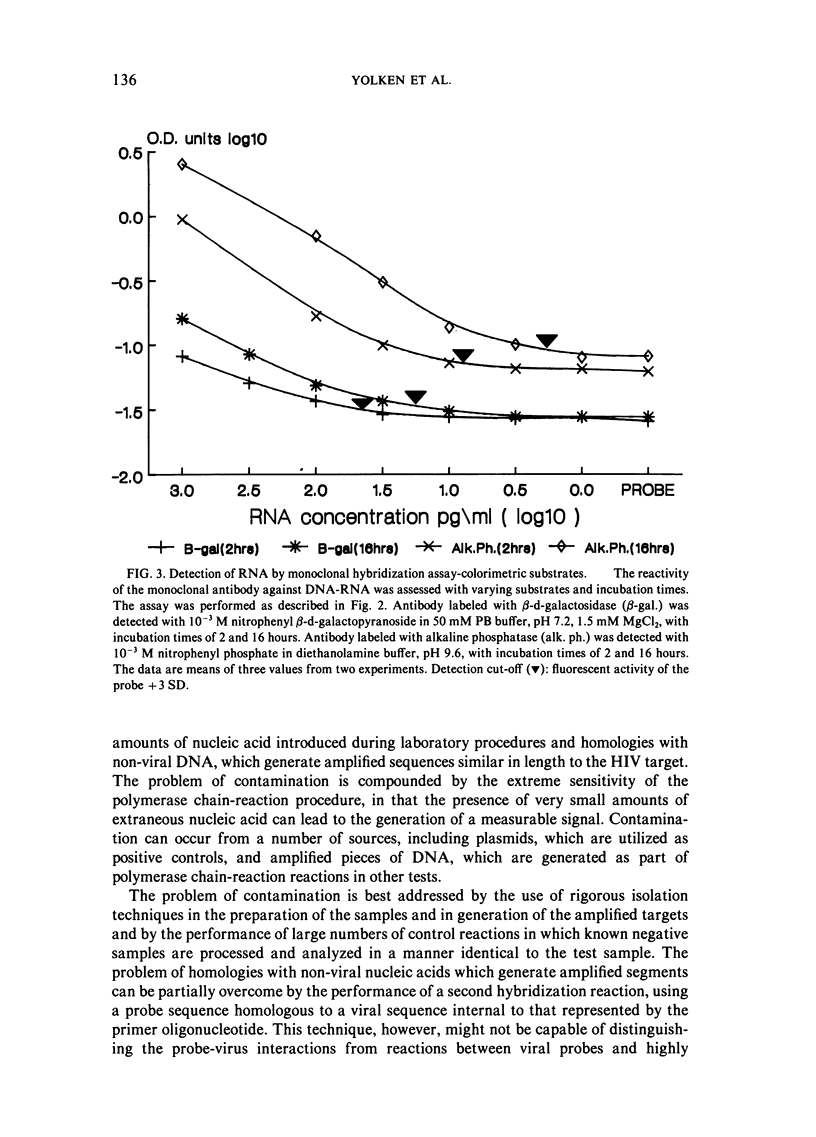



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anh-Tuan N., Novák E. Detection and quantitation of hepatitis-B surface antigen immune complexes (HBsAg-ICs) by an antigen-specific method. I. Detection and quantitation of in vitro prepared HBsAg-ICs. J Immunol Methods. 1980;33(3):293–300. doi: 10.1016/0022-1759(80)90216-1. [DOI] [PubMed] [Google Scholar]
- Boguslawski S. J., Smith D. E., Michalak M. A., Mickelson K. E., Yehle C. O., Patterson W. L., Carrico R. J. Characterization of monoclonal antibody to DNA.RNA and its application to immunodetection of hybrids. J Immunol Methods. 1986 May 1;89(1):123–130. doi: 10.1016/0022-1759(86)90040-2. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carella G., Digeon M., Feldmann G., Jungers P., Drouet J., Bach J. F. Detection of hepatitis B antigen in circulating immune complexes in acute and chronic hepatitis. Scand J Immunol. 1977;6(12):1297–1304. doi: 10.1111/j.1365-3083.1977.tb00369.x. [DOI] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S., Merigan T. C. Rapid detection and quantitation of human cytomegalovirus in urine through DNA hybridization. N Engl J Med. 1983 Apr 21;308(16):921–925. doi: 10.1056/NEJM198304213081603. [DOI] [PubMed] [Google Scholar]
- Demmler G. J., Buffone G. J., Schimbor C. M., May R. A. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988 Dec;158(6):1177–1184. doi: 10.1093/infdis/158.6.1177. [DOI] [PubMed] [Google Scholar]
- Eiden J., Sato S., Yolken R. Specificity of dot hybridization assay in the presence of rRNA for detection of rotaviruses in clinical specimens. J Clin Microbiol. 1987 Sep;25(9):1809–1811. doi: 10.1128/jcm.25.9.1809-1811.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermine A., Tordo N., Tsiang H. Rapid diagnosis of rabies infection by means of a dot hybridization assay. Mol Cell Probes. 1988 Mar;2(1):75–82. doi: 10.1016/0890-8508(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Farzadegan H., Polis M. A., Wolinsky S. M., Rinaldo C. R., Jr, Sninsky J. J., Kwok S., Griffith R. L., Kaslow R. A., Phair J. P., Polk B. F. Loss of human immunodeficiency virus type 1 (HIV-1) antibodies with evidence of viral infection in asymptomatic homosexual men. A report from the Multicenter AIDS Cohort Study. Ann Intern Med. 1988 Jun;108(6):785–790. doi: 10.7326/0003-4819-108-6-785. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Macher A. M., Longo D. L., Lane H. C., Rook A. H., Masur H., Gelmann E. P. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann Intern Med. 1984 Jan;100(1):92–106. doi: 10.7326/0003-4819-100-1-92. [DOI] [PubMed] [Google Scholar]
- Forster A. C., McInnes J. L., Skingle D. C., Symons R. H. Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, photobiotin. Nucleic Acids Res. 1985 Feb 11;13(3):745–761. doi: 10.1093/nar/13.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C., Schochetman G., Spira T., Lifson A., Moore J., Galphin J., Sninsky J., Ou C. Y. Direct detection of HIV RNA expression in seropositive subjects. Lancet. 1988 Sep 10;2(8611):596–599. doi: 10.1016/s0140-6736(88)90639-3. [DOI] [PubMed] [Google Scholar]
- Hsiung G. D. Progress in clinical virology--1960 to 1980: a recollection of twenty years. Yale J Biol Med. 1980 Jan-Feb;53(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laure F., Courgnaud V., Rouzioux C., Blanche S., Veber F., Burgard M., Jacomet C., Griscelli C., Brechot C. Detection of HIV1 DNA in infants and children by means of the polymerase chain reaction. Lancet. 1988 Sep 3;2(8610):538–541. doi: 10.1016/s0140-6736(88)92659-1. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Liang W., Johnson J. P. Rapid plasmid insert amplification with polymerase chain reaction. Nucleic Acids Res. 1988 Apr 25;16(8):3579–3579. doi: 10.1093/nar/16.8.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loche M., Mach B. Identification of HIV-infected seronegative individuals by a direct diagnostic test based on hybridisation to amplified viral DNA. Lancet. 1988 Aug 20;2(8608):418–421. doi: 10.1016/s0140-6736(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Popovic M., Patel A., Veren K., Fladager A., Orndorff S., Gallo R. C. Advances in the isolation of HTLV-III from patients with AIDS and AIDS-related complex and from donors at risk. Cancer Res. 1985 Sep;45(9 Suppl):4588s–4591s. [PubMed] [Google Scholar]
- Matthews J. A., Kricka L. J. Analytical strategies for the use of DNA probes. Anal Biochem. 1988 Feb 15;169(1):1–25. doi: 10.1016/0003-2697(88)90251-5. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Phair J. P., Wolinsky S. Diagnosis of infection with the human immunodeficiency virus. J Infect Dis. 1989 Feb;159(2):320–323. doi: 10.1093/infdis/159.2.320. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Sandberg-Wollheim M., Mettus R. V., Ray P. E., DeFreitas E., Koprowski H. Amplification and molecular cloning of HTLV-I sequences from DNA of multiple sclerosis patients. Science. 1989 Jan 27;243(4890):529–533. doi: 10.1126/science.2536193. [DOI] [PubMed] [Google Scholar]
- Richman D., Schmidt N., Plotkin S., Yolken R., Cherensky M., McIntosh K., Mattheis M. Summary of a workshop on new and useful methods in rapid viral diagnosis. J Infect Dis. 1984 Dec;150(6):941–951. doi: 10.1093/infdis/150.6.941. [DOI] [PubMed] [Google Scholar]
- Sauerbrei A., Wutzler P., Färber I., Brichácek B., Swoboda R., Macheleidt S. Comparative detection of herpesviruses in tissue specimens by in situ hybridization and immunofluorescence. Acta Virol. 1986 May;30(3):213–219. [PubMed] [Google Scholar]
- Schochetman G., Ou C. Y., Jones W. K. Polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1154–1157. doi: 10.1093/infdis/158.6.1154. [DOI] [PubMed] [Google Scholar]
- Schuster V., Matz B., Wiegand H., Polack A., Corsten B., Neumann-Haefelin D. Nucleic acid hybridization for detection of herpes viruses in clinical specimens. J Med Virol. 1986 Jul;19(3):277–286. doi: 10.1002/jmv.1890190310. [DOI] [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Appleman M. D., Causey D. M., Leedom J. M., Arnheim N. Detection of cytomegalovirus DNA in peripheral blood of patients infected with human immunodeficiency virus. J Infect Dis. 1988 Dec;158(6):1185–1192. doi: 10.1093/infdis/158.6.1185. [DOI] [PubMed] [Google Scholar]
- Smith T., Jakobsen J., Gaub J., Helweg-Larsen S., Trojaborg W. Clinical and electrophysiological studies of human immunodeficiency virus-seropositive men without AIDS. Ann Neurol. 1988 Mar;23(3):295–297. doi: 10.1002/ana.410230313. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos D. E., Kappes J. C., Bernstein D. I. Enhanced in vitro reactivation of herpes simplex virus type 2 from latently infected guinea-pig neural tissues by 5-azacytidine. J Gen Virol. 1988 May;69(Pt 5):1079–1083. doi: 10.1099/0022-1317-69-5-1079. [DOI] [PubMed] [Google Scholar]
- Virtanen M., Palva A., Laaksonen M., Halonen P., Söderlund H., Ranki M. Novel test for rapid viral diagnosis: detection of adenovirus in nasopharyngeal mucus aspirates by means of nucleic-acid sandwich hybridisation. Lancet. 1983 Feb 19;1(8321):381–383. doi: 10.1016/s0140-6736(83)91500-3. [DOI] [PubMed] [Google Scholar]
- Viscidi R. P., Connelly C. J., Yolken R. H. Novel chemical method for the preparation of nucleic acids for nonisotopic hybridization. J Clin Microbiol. 1986 Feb;23(2):311–317. doi: 10.1128/jcm.23.2.311-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi R. P., Yolken R. G. Molecular diagnosis of infectious diseases by nucleic acid hybridization. Mol Cell Probes. 1987 Mar;1(1):3–14. doi: 10.1016/0890-8508(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Wilbur D. C., Reichman R. C., Stoler M. H. Detection of infection by human papillomavirus in genital condylomata. A comparison study using immunocytochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988 Apr;89(4):505–510. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]
- Yehle C. O., Patterson W. L., Boguslawski S. J., Albarella J. P., Yip K. F., Carrico R. J. A solution hybridization assay for ribosomal RNA from bacteria using biotinylated DNA probes and enzyme-labeled antibody to DNA:RNA. Mol Cell Probes. 1987 Jun;1(2):177–193. doi: 10.1016/0890-8508(87)90026-0. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Nucleic acids or immunoglobulins: which are the molecular probes of the future? Mol Cell Probes. 1988 Jun;2(2):87–96. doi: 10.1016/0890-8508(88)90030-8. [DOI] [PubMed] [Google Scholar]


