Abstract
CD4+ T cells mediating both delayed-type hypersensitivity (DTH) and acquired cellular resistance (ACR) were generated in mice after immunization with viable Listeria monocytogenes. In contrast, CD4+ T cells from mice immunized with killed L. monocytogenes in complete Freund's adjuvant were capable of mediating only DTH but not ACR. To determine the functional difference between T cells mediating DTH and T cells mediating ACR, we examined two different populations of T cells for profiles of lymphokine production after stimulation with a specific antigen in vitro. The production of interleukin-2 (IL-2) and IL-3 but not IL-4 was observed in both T cells mediating only DTH and those mediating DTH and ACR. In this respect, both types of T cells could be categorized into the TH1 population, and they produced macrophage chemotactic factor equally well. However, the production of gamma interferon (IFN-gamma) was observed only in T cells capable of mediating both DTH and ACR. This result was confirmed not only by an enzyme immunoassay specific for murine IFN-gamma but also by Northern (RNA) analysis for the detection of IFN-gamma mRNA. These results suggested that the TH1 population may be subdivided further into two distinct subsets and that the ineffectiveness of the killed bacterial vaccine may be partly explained by the dissociated development of T cell function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. R., Black W. J., Cannon J. G. Neisserial antigen H.8 is immunogenic in patients with disseminated gonococcal and meningococcal infections. J Infect Dis. 1985 Apr;151(4):650–657. doi: 10.1093/infdis/151.4.650. [DOI] [PubMed] [Google Scholar]
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Woan M., Sajewski D. H., McGregor D. D. T-cell co-operation in the mediation of acquired resistance to Listeria monocytogenes. Immunology. 1985 Sep;56(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J. Administration of purified anti-L3T4 monoclonal antibody impairs the resistance of mice to Listeria monocytogenes infection. Infect Immun. 1989 Jan;57(1):100–109. doi: 10.1128/iai.57.1.100-109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Henson P. M., Campbell P. A. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J Leukoc Biol. 1984 Feb;35(2):193–208. doi: 10.1002/jlb.35.2.193. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
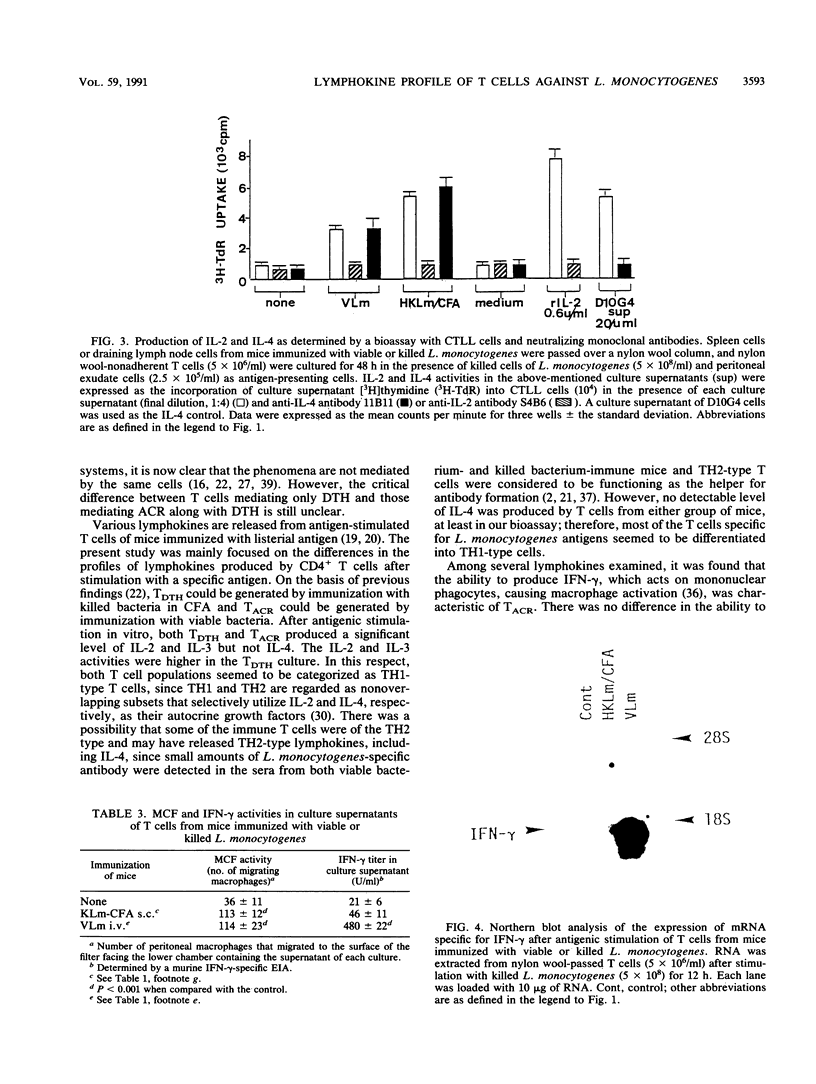
- Handa T., Mitsuyama M., Serushago B. A., Muramori K., Nomoto K. Co-operative effect of MCF and MAF(IFN-gamma) in the protection of mice against Listeria monocytogenes. Immunology. 1988 Nov;65(3):427–432. [PMC free article] [PubMed] [Google Scholar]
- Handa T., Mitsuyama M., Watanabe Y., Koga T., Nomoto K. A significant role of the macrophage accumulation induced by MCF in the protection of mice against Listeria monocytogenes in vivo. Cell Immunol. 1987 May;106(2):330–342. doi: 10.1016/0008-8749(87)90176-6. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Mitsuyama M., Muramori K., Tsukada H., Nomoto K. Interleukin-1-induced promotion of T-cell differentiation in mice immunized with killed Listeria monocytogenes. Infect Immun. 1990 Dec;58(12):3973–3979. doi: 10.1128/iai.58.12.3973-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Rebar L., Keller J., Lee J. C., Hapel A. J. Interleukin 3: possible roles in the regulation of lymphocyte differentiation and growth. Immunol Rev. 1982;63:5–32. doi: 10.1111/j.1600-065x.1982.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Carding S., Jones B., Murray J., Portoles P., Rasmussen R., Rojo J., Saizawa K., West J., Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988 Jan;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Gill T. J., 3rd, Kunz H. W., Jungi R. Genetic control of cell-mediated immunity in the rat. III. T cells restricted by the RT1.A locus recognize viable Listeria but not isolated bacterial antigens. J Immunogenet. 1982 Dec;9(6):445–456. doi: 10.1111/j.1744-313x.1982.tb01006.x. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Generation of macrophage chemotactic activity in situ in Listeria-immune rats. Cell Immunol. 1977 Oct;33(2):322–339. doi: 10.1016/0008-8749(77)90162-9. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Simon M. M., Röllinghoff M., Wagner H. Interleukin 2 induction in Lyt 1+ 23- T cells from Listeria monocytogenes-immune mice. Infect Immun. 1982 Sep;37(3):1292–1294. doi: 10.1128/iai.37.3.1292-1294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Koga T., Mitsuyama M., Handa T., Yayama T., Muramori K., Nomoto K. Induction by killed Listeria monocytogenes of effector T cells mediating delayed-type hypersensitivity but not protection in mice. Immunology. 1987 Oct;62(2):241–248. [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. E., Ehlers S., Hahn H. T-cell subsets in delayed-type hypersensitivity, protection, and granuloma formation in primary and secondary Listeria infection in mice: superior role of Lyt-2+ cells in acquired immunity. Infect Immun. 1988 Aug;56(8):1920–1925. doi: 10.1128/iai.56.8.1920-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Handa T., Koga T., Watanabe Y., Yayama T., Muramori K., Nomoto K. In vitro primary induction of T cells mediating delayed footpad reaction and acquired cellular resistance to Listeria monocytogenes. Immunobiology. 1988 Jul;177(3):254–266. doi: 10.1016/S0171-2985(88)80045-7. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M., Igarashi K., Kawamura I., Ohmori T., Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990 May;58(5):1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Nomoto K., Takeya K. Direct correlation between delayed footpad reaction and resistance to local bacterial infection. Infect Immun. 1982 Apr;36(1):72–79. doi: 10.1128/iai.36.1.72-79.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Watanabe Y., Sano M., Amako K., Nomoto K. Generation of Listeria monocytogenes-specific T cells mediating delayed footpad reaction and protection in neonatally thymectomized mice but not in nude mice. Med Microbiol Immunol. 1988;177(4):207–217. doi: 10.1007/BF00211220. [DOI] [PubMed] [Google Scholar]
- Miyata M., Mitsuyama M., Ogata N., Nomoto K., Takeya K. Two steps in the generation of acquired cellular resistance against Listeria monocytogenes: accumulation and activation of macrophages. Immunology. 1982 Oct;47(2):247–253. [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Sperling U., Kaufmann S. H., Hahn H. Production of macrophage-activating and migration-inhibition factors in vitro by serologically selected and cloned Listeria monocytogenes-specific T cells of the Lyt 1+2- phenotype. Infect Immun. 1984 Oct;46(1):111–115. doi: 10.1128/iai.46.1.111-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- von Koenig C. H., Finger H., Hof H. Failure of killed Listeria monocytogenes vaccine to produce protective immunity. Nature. 1982 May 20;297(5863):233–234. doi: 10.1038/297233a0. [DOI] [PubMed] [Google Scholar]



