Abstract
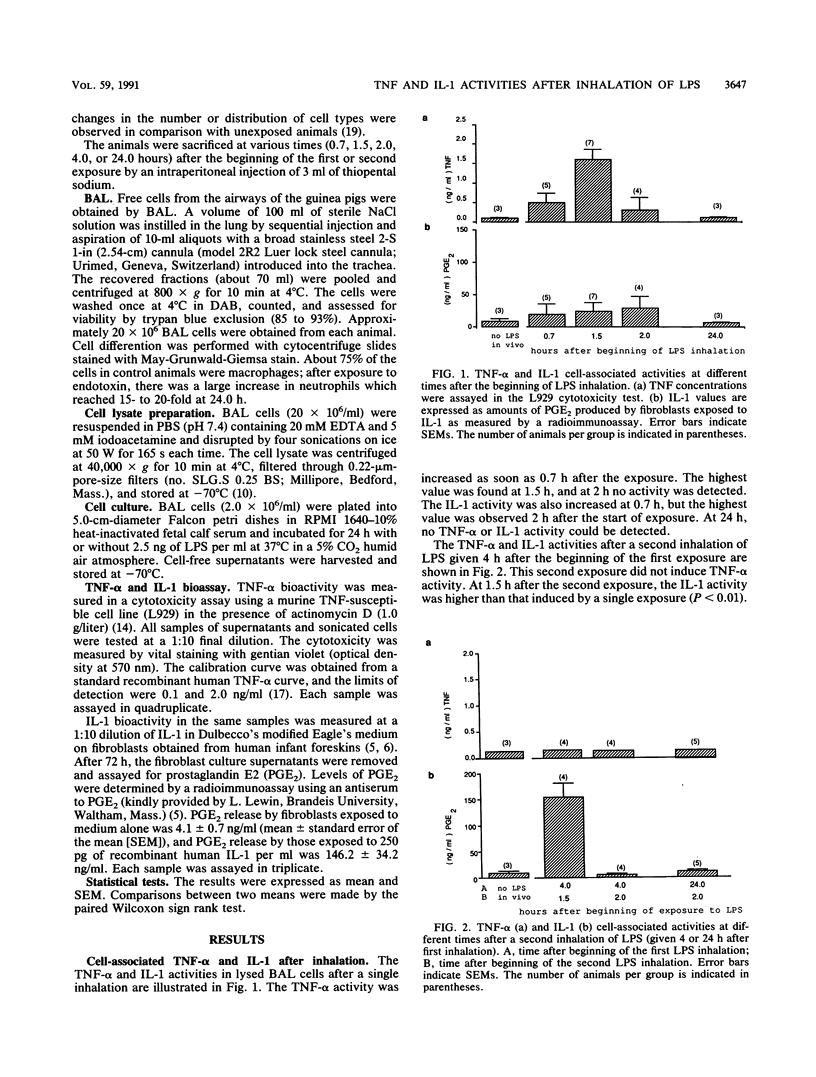
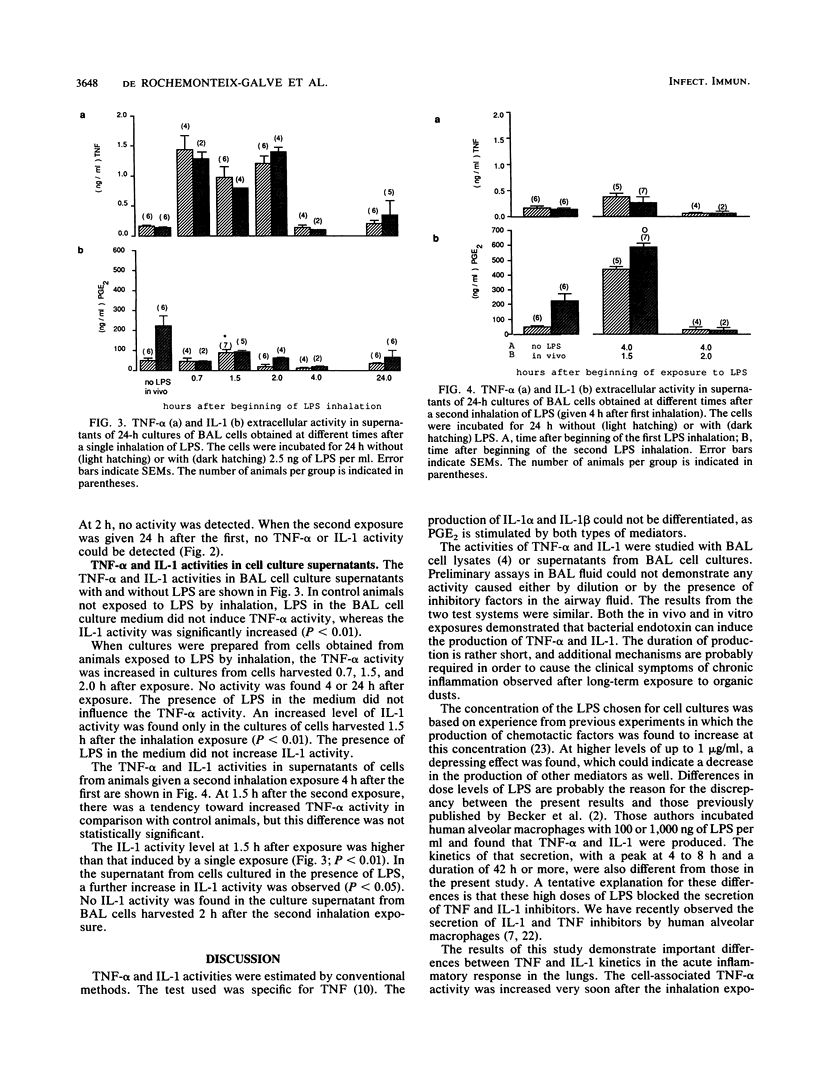
Bacterial endotoxins (lipopolysaccharides), important components of many organic dusts, are known to induce macrophages to produce the inflammatory mediators interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-alpha). To investigate the role of these mediators in the early inflammatory responses in the lung, guinea pigs were exposed to an aerosol of bacterial endotoxin. A bronchoalveolar lavage (BAL) was then performed, and TNF-alpha and IL-1 in lysed BAL cells and in the supernatants from BAL cell cultures were studied. The effect of single and repeated LPS inhalation exposures on the activities of TNF and IL-1 was studied, as was the effect of LPS added to the cell culture medium. A single inhalation exposure to LPS caused an increase in the TNF-alpha and IL-1 activities in cell lysate and in the cell culture supernatant. After a second inhalation exposure, cell-associated and extracellular TNF-alpha activity could not be detected, whereas IL-1 activity was markedly enhanced. IL-1 activity was increased when LPS was added to the cell culture medium with or without a prior inhalation exposure. In contrast, TNF-alpha activity was not affected after a second exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauss F., Dröge W., Männel D. N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987 Jul;55(7):1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Devlin R. B., Haskill J. S. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J Leukoc Biol. 1989 Apr;45(4):353–361. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Remick D. G., Shmyr-Forsch C., Beals T. F., Kunkel S. L. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988 Dec;133(3):564–572. [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Sundström L., Polla B. S., Junod A. F. Cultured human alveolar macrophages from smokers with lung cancer: resolution of factors that stimulate fibroblast proliferation, production of collagenase, or prostaglandin E2. J Leukoc Biol. 1985 May;37(5):641–649. doi: 10.1002/jlb.37.5.641. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Hauser C., Dayer J. M., Jaunin F., de Rochemonteix B., Saurat J. H. Intracellular epidermal interleukin 1-like factors in the human epidermoid carcinoma cell line A431. Cell Immunol. 1986 Jun;100(1):89–96. doi: 10.1016/0008-8749(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Heicappell R., Naito S., Ichinose Y., Creasey A. A., Lin L. S., Fidler I. J. Cytostatic and cytolytic effects of human recombinant tumor necrosis factor on human renal cell carcinoma cell lines derived from a single surgical specimen. J Immunol. 1987 Mar 1;138(5):1634–1640. [PubMed] [Google Scholar]
- Henderson V., Enterline P. E. An unusual mortality experience in cotton textile workers. J Occup Med. 1973 Sep;15(9):717–719. [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J. H., Jensen O. M. Occupation and risk of cancer in Denmark. An analysis of 93,810 cancer cases, 1970-1979. Scand J Work Environ Health. 1987;13 (Suppl 1):1–91. [PubMed] [Google Scholar]
- Organic dusts and lung diseases. Proceedings of an international workshop. Skokloster, Sweden, October 24-27, 1988. Am J Ind Med. 1990;17(1):1–148. [PubMed] [Google Scholar]
- Rylander R., Fogelmark B., Sjöstrand M. Free lung cell phagocytosis and lysosomal enzyme activity after inhalation of lipopolysaccharide in guinea-pigs. Agents Actions. 1985 Jul;16(5):353–358. doi: 10.1007/BF01982872. [DOI] [PubMed] [Google Scholar]
- Rylander R. The role of endotoxin for reactions after exposure to cotton dust. Am J Ind Med. 1987;12(6):687–697. doi: 10.1002/ajim.4700120607. [DOI] [PubMed] [Google Scholar]
- Seckinger P., Isaaz S., Dayer J. M. A human inhibitor of tumor necrosis factor alpha. J Exp Med. 1988 Apr 1;167(4):1511–1516. doi: 10.1084/jem.167.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snella M. C., Rylander R. Lung cell reactions after inhalation of bacterial lipopolysaccharides. Eur J Respir Dis. 1982 Nov;63(6):550–557. [PubMed] [Google Scholar]
- Tabor D. R., Burchett S. K., Jacobs R. F. Enhanced production of monokines by canine alveolar macrophages in response to endotoxin-induced shock. Proc Soc Exp Biol Med. 1988 Apr;187(4):408–415. doi: 10.3181/00379727-187-42681. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- de Rochemonteix-Galve B., Dayer J. M., Junod A. F. Fibroblast-alveolar cell interactions in sarcoidosis and idiopathic pulmonary fibrosis: evidence for stimulatory and inhibitory cytokine production by alveolar cells. Eur Respir J. 1990 Jun;3(6):653–664. [PubMed] [Google Scholar]



