Abstract
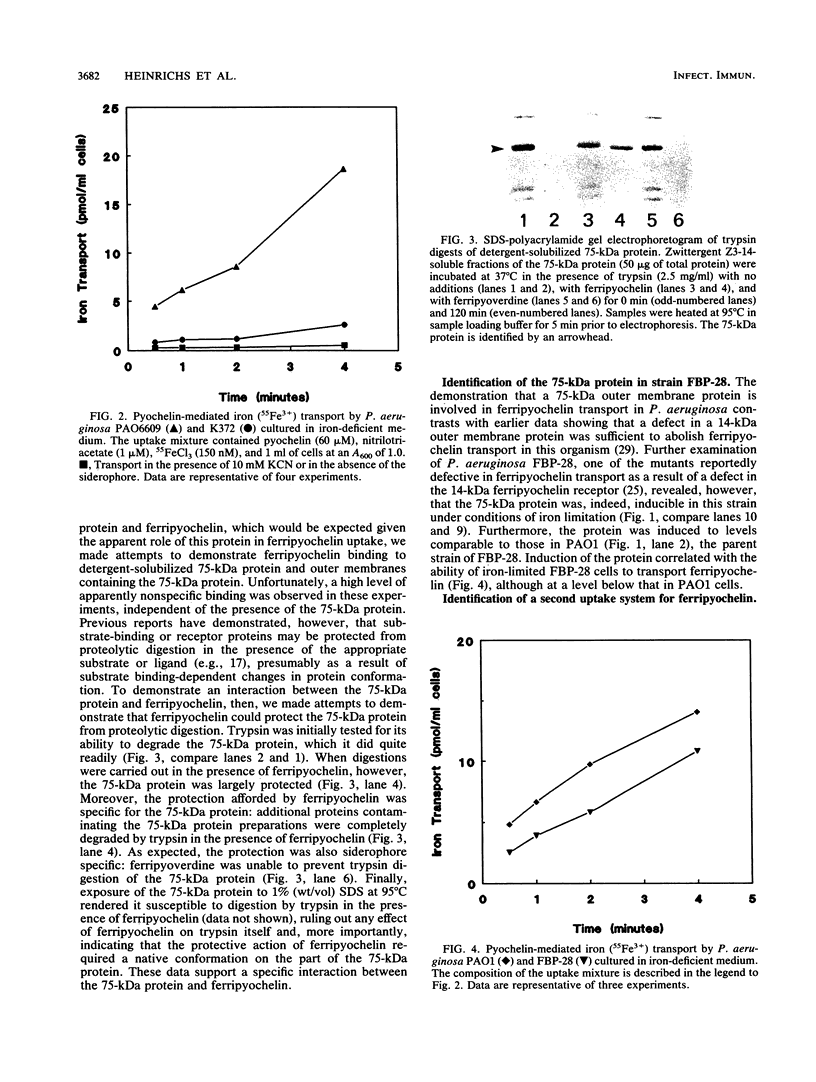
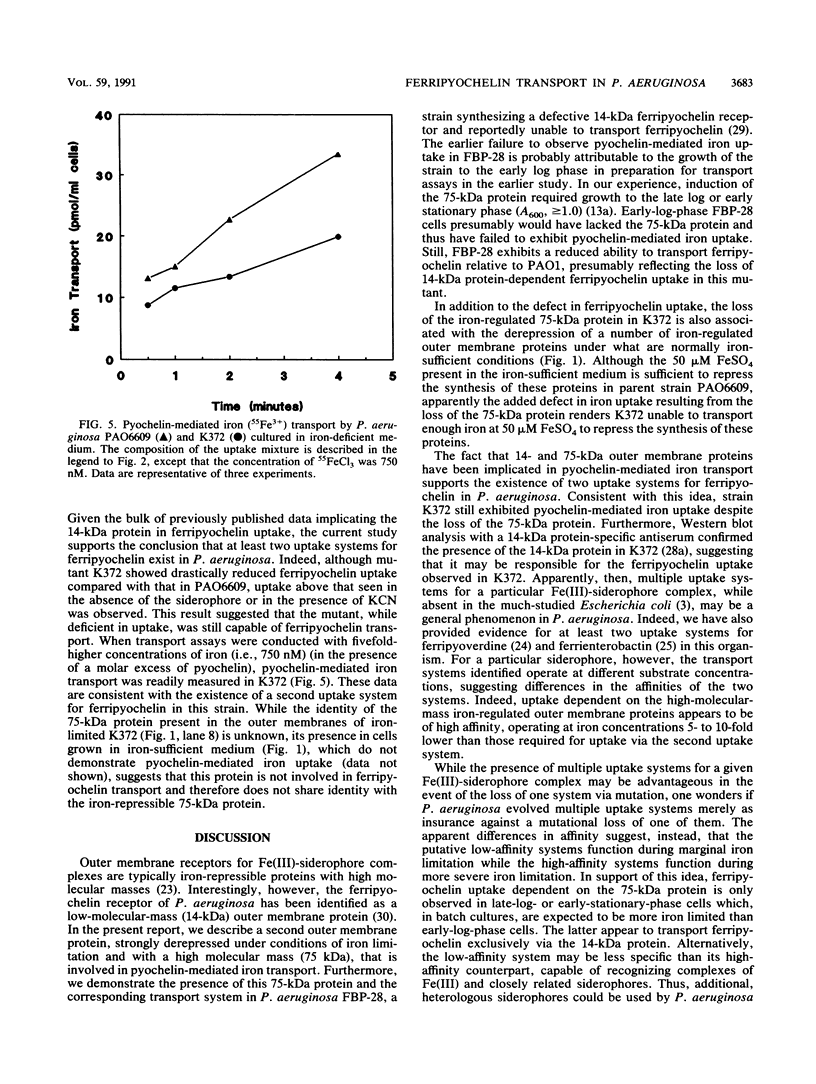
An iron-regulated outer membrane protein of 75,000 daltons was strongly expressed following iron limitation of strains of Pseudomonas aeruginosa which fail to produce pyoverdine. A mutant nonderepressible for this protein (K372) was deficient in pyochelin-mediated iron transport at 150 nM FeCl3, consistent with a role for the 75-kDa protein in ferripyochelin transport. Moreover, ferripyochelin specifically protected the 75-kDa protein against trypsin digestion, supporting an interaction between ferripyochelin and the 75-kDa protein. Previous reports implicated a 14,000-dalton outer membrane protein as the receptor for ferripyochelin (P.A. Sokol and D.E. Woods, Infect. Immun. 40:665-669, 1983) and demonstrated that a mutant (FBP-28) expressing a defective 14-kDa outer membrane protein did not exhibit pyochelin-mediated iron transport (P.A. Sokol, J. Bacteriol. 169:3365-3368, 1987). Nonetheless, we were able to demonstrate (i) that FBP-28 was inducible for the 75-kDa protein under iron-limiting conditions and (ii) that concomitant with the induction of this protein in FBP-28, pyochelin-mediated iron uptake at 150 nM FeCl3 was observed. Interestingly, strain K372 did transport ferripyochelin at higher (750 nM) FeCl3 concentrations, suggesting that a second pyochelin-mediated iron transport system, perhaps involving the 14-kDa outer membrane protein identified previously, operates in P. aeruginosa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer R. G., Toyokuni T., Staley A., Rinehart K. L., Jr, Cox C. D. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol. 1988 Nov;170(11):5344–5351. doi: 10.1128/jb.170.11.5344-5351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzenhart K., Rüden H. Hospital infections caused by Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:1–15. doi: 10.1159/000414328. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Moguilevsky N., Jacques J. F., Masson P. L. Study of the siderophores and receptors in different clinical isolates of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:290–306. doi: 10.1159/000414354. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982 Apr;36(1):17–23. doi: 10.1128/iai.36.1.17-23.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Konopka K., Bindereif A., Neilands J. B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982 Dec 7;21(25):6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron (III) hydroxamate transport into Escherichia coli. Substrate binding to the periplasmic FhuD protein. J Biol Chem. 1990 Dec 15;265(35):21407–21410. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Hohnadel D., Hallé F. Cepabactin from Pseudomonas cepacia, a new type of siderophore. J Gen Microbiol. 1989 Jun;135(6):1479–1487. doi: 10.1099/00221287-135-6-1479. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Hohnadel D., Khan A., Cornelis P. Pyoverdin-facilitated iron uptake in Pseudomonas aeruginosa: immunological characterization of the ferripyoverdin receptor. Mol Microbiol. 1990 Aug;4(8):1401–1405. doi: 10.1111/j.1365-2958.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Poole K., Neshat S., Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991 Feb;62(1):1–5. [PubMed] [Google Scholar]
- Poole K., Young L., Neshat S. Enterobactin-mediated iron transport in Pseudomonas aeruginosa. J Bacteriol. 1990 Dec;172(12):6991–6996. doi: 10.1128/jb.172.12.6991-6996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A. Tn5 insertion mutants of Pseudomonas aeruginosa deficient in surface expression of ferripyochelin-binding protein. J Bacteriol. 1987 Jul;169(7):3365–3368. doi: 10.1128/jb.169.7.3365-3368.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Characterization of antibody to the ferripyochelin-binding protein of Pseudomonas aeruginosa. Infect Immun. 1986 Mar;51(3):896–900. doi: 10.1128/iai.51.3.896-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriyosachati S., Cox C. D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986 Jun;52(3):885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Breeding S. A., Lankford C. E. Enterochelin (enterobactin): virulence factor for Salmonella typhimurium. Infect Immun. 1979 Apr;24(1):174–180. doi: 10.1128/iai.24.1.174-180.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]





