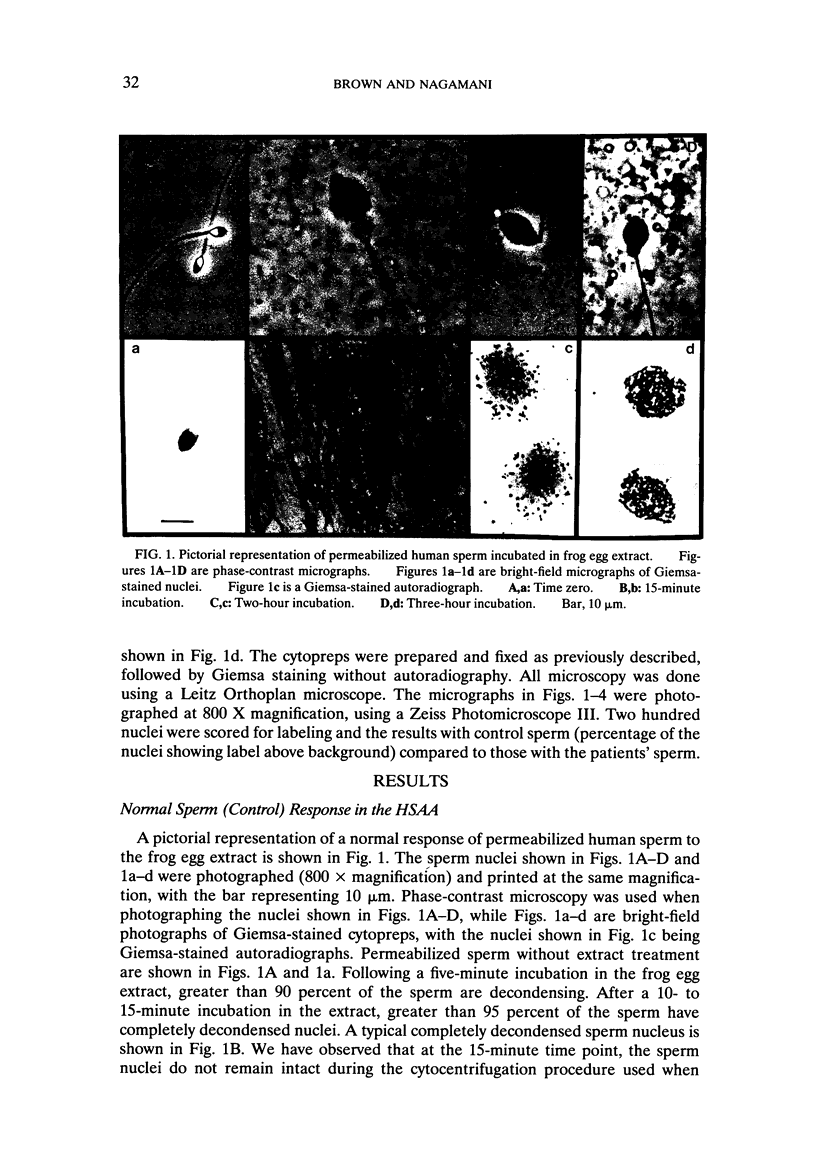
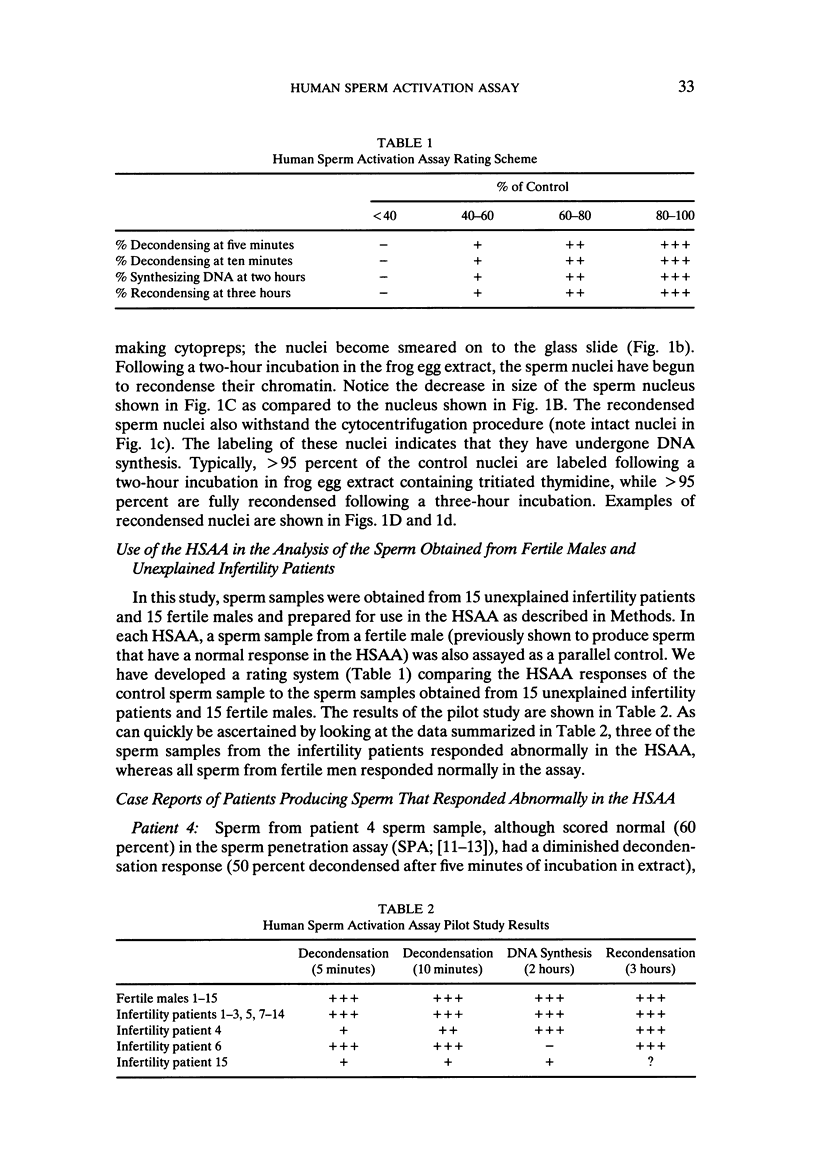
Abstract
Approximately one in six married couples find themselves involuntarily infertile. This ratio translates to between two and four million U.S. couples. Although numerous tests are available for diagnosing infertility problems, 5-10 percent of all couples who seek medical treatment are diagnosed with unexplained infertility. Several tests are presently available for diagnosing male infertility; however, none of the present procedures test for activation of the sperm nucleus following entry into the fertilized egg, a series of events critical for the entry of the zygote into the developmental program. We have developed an in vitro human sperm activation assay, using Xenopus laevis frog egg extract. When normal human sperm is permeabilized and then mixed with frog egg extract, the sperm nuclei decondense, synthesize DNA, and recondense during a three-hour time course. We have tested this assay's utility in diagnosing previously unexplained infertility. We found that 20 percent of the male infertility patients produced sperm that responded abnormally in the assay (95 percent confidence interval, 4-48 percent; n = 15), while sperm samples from 15 fertile males showed no abnormal responses (p = 0.0112). These preliminary results indicate that the human sperm activation assay may be a useful tool for diagnosing some cases of human infertility.
Full text
PDF



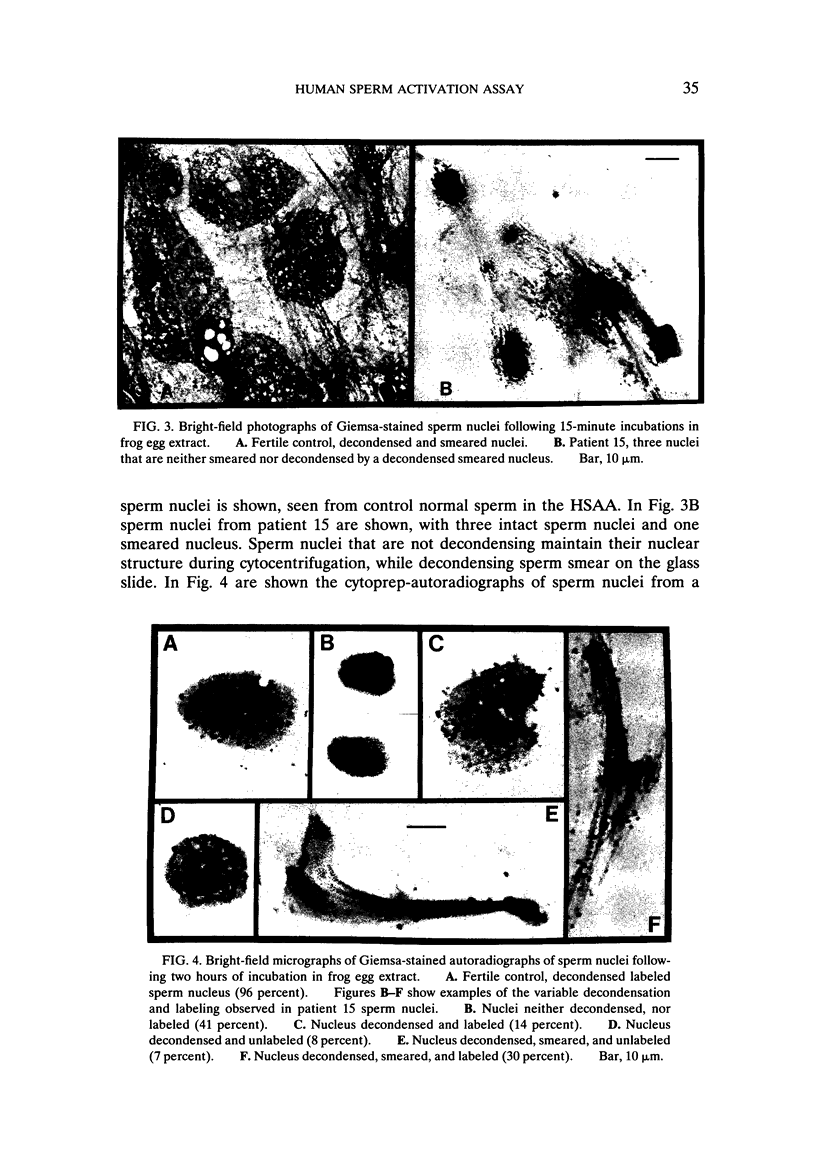



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. B., Blake E. J., Wolgemuth D. J., Gordon K., Ruddle F. H. Chromatin decondensation and DNA synthesis in human sperm activated in vitro by using Xenopus laevis egg extracts. J Exp Zool. 1987 May;242(2):215–231. doi: 10.1002/jez.1402420213. [DOI] [PubMed] [Google Scholar]
- Gordon K., Brown D. B., Ruddle F. H. In vitro activation of human sperm induced by amphibian egg extract. Exp Cell Res. 1985 Apr;157(2):409–418. doi: 10.1016/0014-4827(85)90126-0. [DOI] [PubMed] [Google Scholar]
- Kasinsky H. E., Huang S. Y., Mann M., Roca J., Subirana J. A. On the diversity of sperm histones in the vertebrates: IV. Cytochemical and amino acid analysis in Anura. J Exp Zool. 1985 Apr;234(1):33–46. doi: 10.1002/jez.1402340106. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983 May 13;220(4598):719–721. doi: 10.1126/science.6601299. [DOI] [PubMed] [Google Scholar]
- Longo F. J. Fertilization: a comparative ultrastructural review. Biol Reprod. 1973 Sep;9(2):149–215. doi: 10.1093/biolreprod/9.2.149. [DOI] [PubMed] [Google Scholar]
- Longo F. J. Transformations of sperm nuclei upon insemination. Curr Top Dev Biol. 1978;12:149–184. doi: 10.1016/s0070-2153(08)60596-7. [DOI] [PubMed] [Google Scholar]
- Overstreet J. W., Yanagimachi R., Katz D. F., Hayashi K., Hanson F. W. Penetration of human spermatozoa into the human zona pellucida and the zona-free hamster egg: a study of fertile donors and infertile patients. Fertil Steril. 1980 May;33(5):534–542. doi: 10.1016/s0015-0282(16)44720-5. [DOI] [PubMed] [Google Scholar]
- Perreault S. D., Wolff R. A., Zirkin B. R. The role of disulfide bond reduction during mammalian sperm nuclear decondensation in vivo. Dev Biol. 1984 Jan;101(1):160–167. doi: 10.1016/0012-1606(84)90126-x. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Yanagimachi H., Rogers B. J. The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976 Nov;15(4):471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]