Abstract
We synthesized Cryptococcus neoformans serotype A glucuronoxylomannan (GXM) conjugate vaccines under conditions suitable for human use to prevent disseminated cryptococcosis. The purified, sonicated GXM was derivatized with adipic acid dihydrazide through either hydroxyl or carboxyl groups and then covalently bound to tetanus toxoid (TT) or Pseudomonas aeruginosa exoprotein A (rEPA). The immunogenicity of these conjugates was evaluated in BALB/c and general purpose mice by subcutaneous injection in saline. The conjugates elicited higher GXM antibody responses than GXM alone. Booster immunoglobulin G (IgG) and IgM responses were elicited by all conjugates in BALB/c mice. The conjugates prepared through hydroxyl activation (GXM-TT2 and GXM-rEPA) were more immunogenic than the one prepared through carboxyl activation (GXM-TT1). GXM antibody response was enhanced by the administration of monophosphoryl lipid A 2 days following the injection of GXM-TT2 (P less than 0.03). The conjugates also elicited IgG antibodies to the carrier proteins. Gel diffusion tests using conjugate-induced hyperimmune sera and chemically modified GXMs suggested that the specificity of GXM-TT1-induced antibodies was conferred by the O-acetyl groups. Hyperimmune sera generated by GXM-TT2 precipitated with the chemically unmodified and the de-O-acetylated GXMs but not with the carboxyl-reduced and de-O-acetylated GXM. GXM-TT2-induced hyperimmune serum also precipitated with the capsular polysaccharides of C. neoformans serotypes D, B, and C. The conjugate vaccines prepared through hydroxyl activation of the GXM are sufficiently immunogenic and appear to be suitable for clinical evaluation.
Full text
PDF

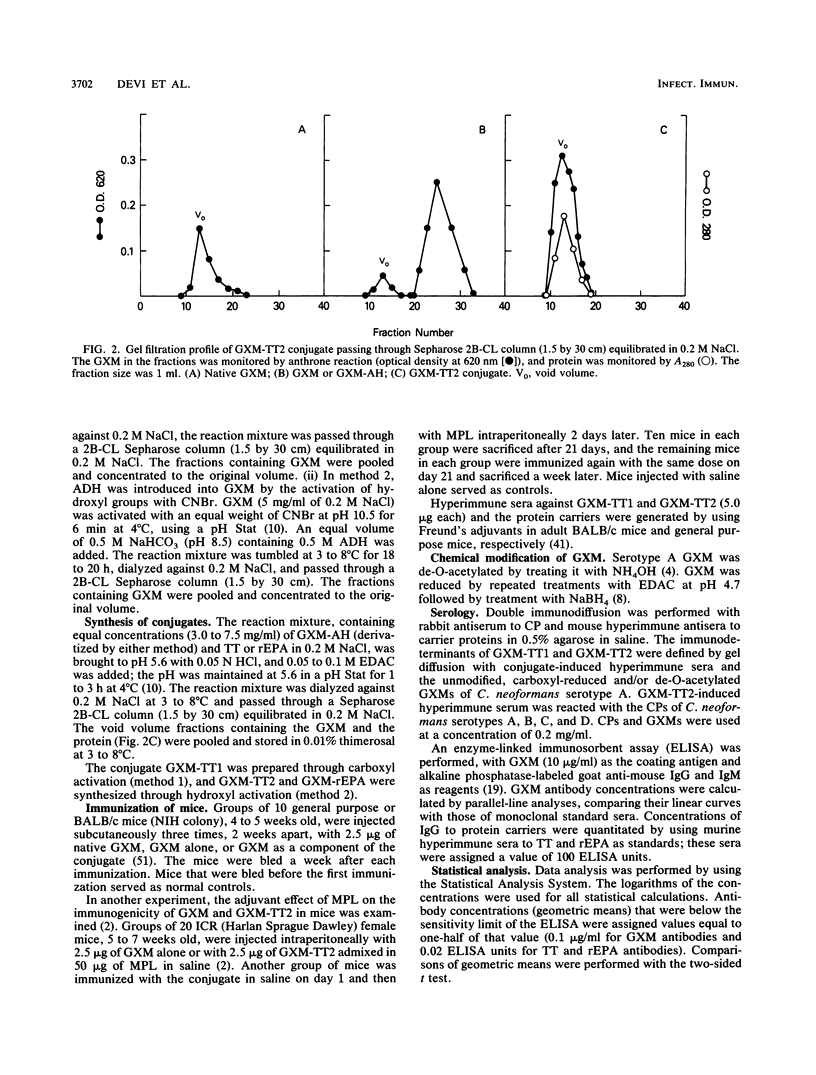
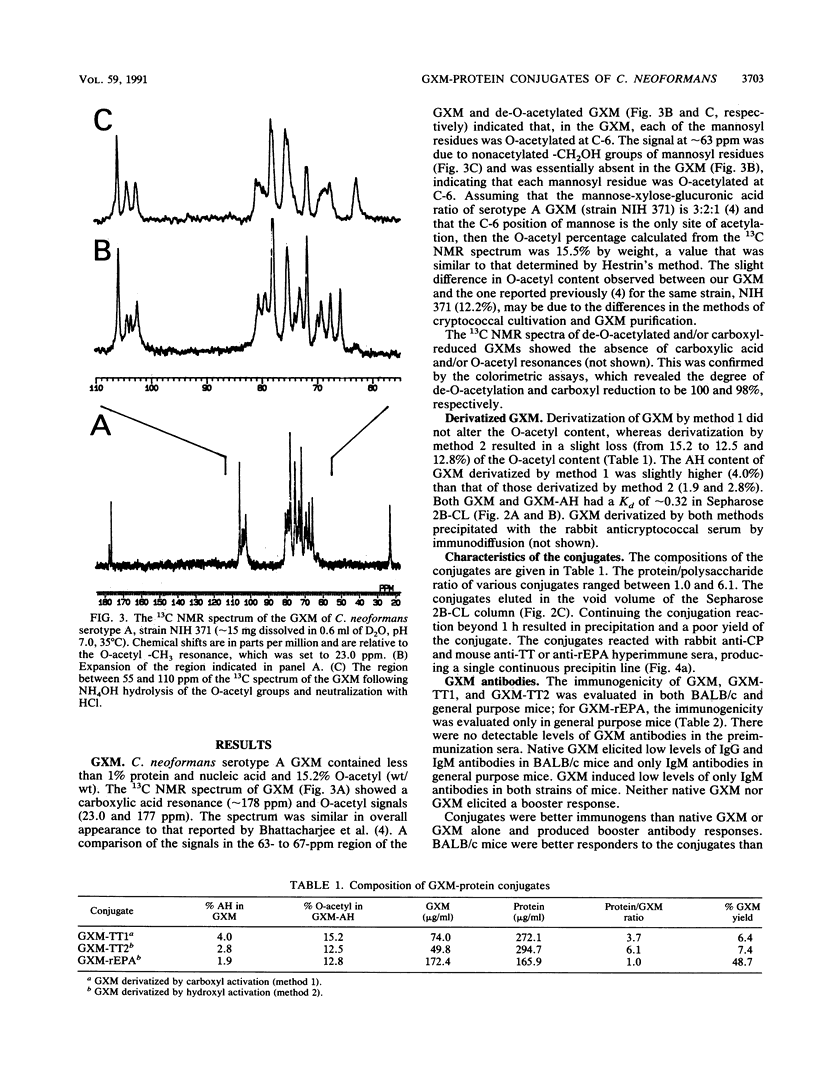
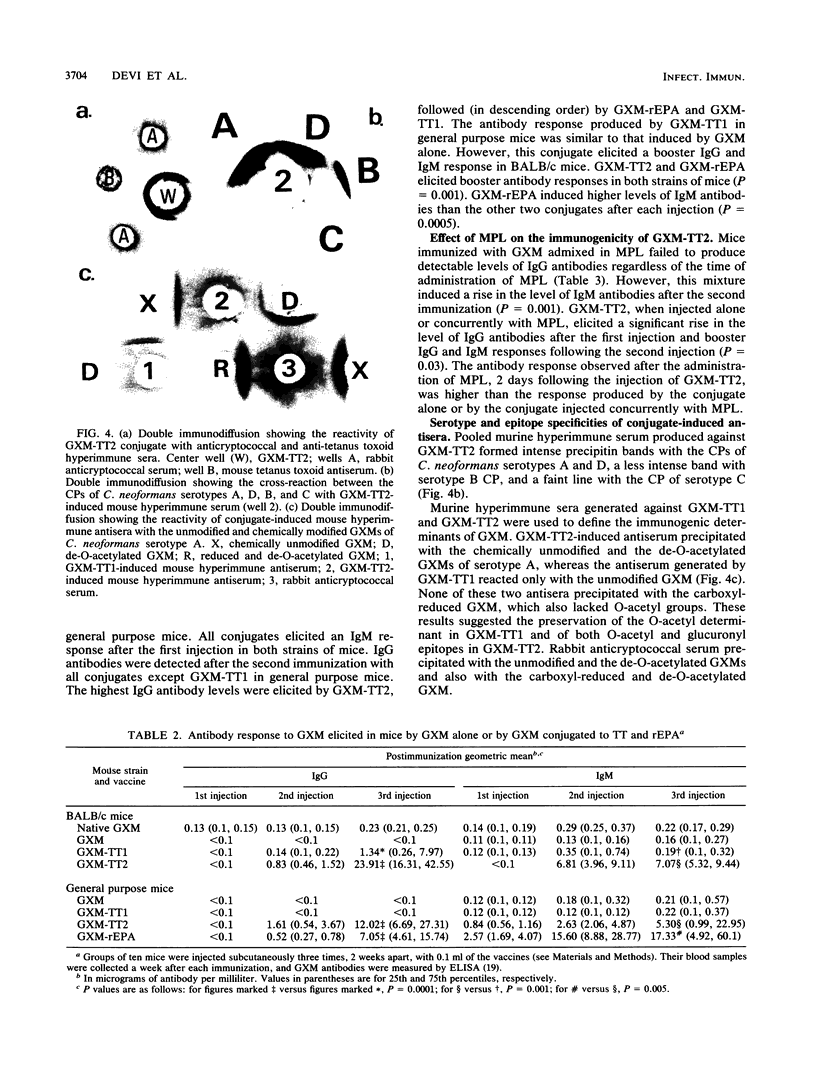



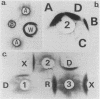
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT J. E., HASENCLEVER H. F., TYNES B. S. DETECTION OF CRYPTOCOCCAL POLYSACCHARIDE IN SERUM AND SPINAL FLUID: VALUE IN DIAGNOSIS AND PROGNOSIS. Trans Assoc Am Physicians. 1964;77:145–150. [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Stashak P. W., Prescott B., Cantrell J. L., Rudbach J. A. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect Immun. 1988 Dec;56(12):3064–3066. doi: 10.1128/iai.56.12.3064-3066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Capsular polysaccharides from a parent strain and from a possible, mutant strain of Cryptococcus neoformans serotype A. Carbohydr Res. 1981 Sep 16;95(2):237–248. doi: 10.1016/s0008-6215(00)85580-9. [DOI] [PubMed] [Google Scholar]
- Bindschadler D. D., Bennett J. E. Serology of human cryptococcosis. Ann Intern Med. 1968 Jul;69(1):45–52. doi: 10.7326/0003-4819-69-1-45. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Scharff M. D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991 Jul 1;174(1):151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R., Jones R. G., Reiss E. Structure determination of Cryptococcus neoformans serotype A-variant glucuronoxylomannan by 13C-n.m.r. spectroscopy. Carbohydr Res. 1988 Jan 15;172(1):113–138. doi: 10.1016/s0008-6215(00)90846-2. [DOI] [PubMed] [Google Scholar]
- Cherniak R., Reiss E., Slodki M. E., Plattner R. D., Blumer S. O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980 Aug;17(8):1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988 Apr;157(4):624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- Dromer F., Aucouturier P., Clauvel J. P., Saimot G., Yeni P. Cryptococcus neoformans antibody levels in patients with AIDS. Scand J Infect Dis. 1988;20(3):283–285. doi: 10.3109/00365548809032452. [DOI] [PubMed] [Google Scholar]
- Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987 Mar;55(3):749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991 May;163(5):1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989 Dec;78(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Salamero J., Contrepois A., Carbon C., Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Mar;55(3):742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Yeni P., Charreire J. Genetic control of the humoral response to cryptococcal capsular polysaccharide in mice. Immunogenetics. 1988;28(6):417–424. doi: 10.1007/BF00355373. [DOI] [PubMed] [Google Scholar]
- Eckert T. F., Kozel T. R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Active immunization against Cryptococcus neoformans. J Infect Dis. 1958 May-Jun;102(3):219–226. doi: 10.1093/infdis/102.3.219. [DOI] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958 Jul;98(3):611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- GORDON M. A., LAPA E. SERUM PROTEIN ENHANCEMENT OF ANTIBIOTIC THERAPY IN CRYPTOCOCCOSIS. J Infect Dis. 1964 Oct;114:373–377. doi: 10.1093/infdis/114.4.373. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–922. [PubMed] [Google Scholar]
- Goren M. B., Middlebrook G. M. Protein conjugates of polysaccharide from Cryptococcus neoforman s. J Immunol. 1967 May;98(5):901–913. [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Henderson D. K., Bennett J. E., Huber M. A. Long-lasting, specific immunologic unresponsiveness associated with cryptococcal meningitis. J Clin Invest. 1982 May;69(5):1185–1190. doi: 10.1172/JCI110555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs M. M., Perfect J. R., Granger D. L., Durack D. T. Opsonic activity of cerebrospinal fluid in experimental cryptococcal meningitis. Infect Immun. 1990 Jul;58(7):2115–2119. doi: 10.1128/iai.58.7.2115-2119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Simmons R. L., Najarian J. S. Fungal infections in renal transplant recipients. Ann Surg. 1978 Nov;188(5):598–605. doi: 10.1097/00000658-197811000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. H., Rosen P. P., Armstrong D. Cryptococcosis in a cancer hospital: clinical and pathological correlates in forty-six patients. Cancer. 1977 May;39(5):2265–2274. doi: 10.1002/1097-0142(197705)39:5<2265::aid-cncr2820390546>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gulley W. F., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977 Dec;18(3):701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Role of the capsule in phagocytosis of Cryptococcus neoformans. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S436–S439. doi: 10.1093/cid/10.supplement_2.s436. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984 Jul;120(1):123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- Lagergard T., Shiloach J., Robbins J. B., Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990 Mar;58(3):687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Farrell T. P., Maziarz R. T. Killing of Cryptococcus neoformans by human peripheral blood mononuclear cells stimulated in culture. J Infect Dis. 1991 May;163(5):1108–1113. doi: 10.1093/infdis/163.5.1108. [DOI] [PubMed] [Google Scholar]
- Lewis J. L., Rabinovich S. The wide spectrum of cryptococcal infections. Am J Med. 1972 Sep;53(3):315–322. doi: 10.1016/0002-9343(72)90174-x. [DOI] [PubMed] [Google Scholar]
- Miller G. P., Kohl S. Antibody-dependent leukocyte killing of Cryptococcus neoformans. J Immunol. 1983 Sep;131(3):1455–1459. [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun. 1986 Feb;51(2):556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E., Cherniak R., Eby R., Kaufman L. Enzyme immunoassay detection of IgM to galactoxylomannan of Cryptococcus neoformans. Diagn Immunol. 1984;2(2):109–115. [PubMed] [Google Scholar]
- Sanford J. E., Lupan D. M., Schlageter A. M., Kozel T. R. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun. 1990 Jun;58(6):1919–1923. doi: 10.1128/iai.58.6.1919-1923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlageter A. M., Kozel T. R. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun. 1990 Jun;58(6):1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. N., Muchmore H. G., Felton F. G. Enzyme-linked immunosorbent assays in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):257–265. doi: 10.1080/00362178185380431. [DOI] [PubMed] [Google Scholar]
- Szu S. C., Zon G., Schneerson R., Robbins J. B. Ultrasonic irradiation of bacterial polysaccharides. Characterization of the depolymerized products and some applications of the process. Carbohydr Res. 1986 Sep 1;152:7–20. doi: 10.1016/s0008-6215(00)90283-0. [DOI] [PubMed] [Google Scholar]
- Todaro-Luck F., Reiss E., Cherniak R., Kaufman L. Characterization of Cryptococcus neoformans capsular glucuronoxylomannan polysaccharide with monoclonal antibodies. Infect Immun. 1989 Dec;57(12):3882–3887. doi: 10.1128/iai.57.12.3882-3887.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. E., Bennett J. E., Bailey J. W. Serologic grouping of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1968 Mar;127(3):820–823. doi: 10.3181/00379727-127-32812. [DOI] [PubMed] [Google Scholar]