Abstract
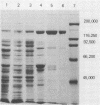
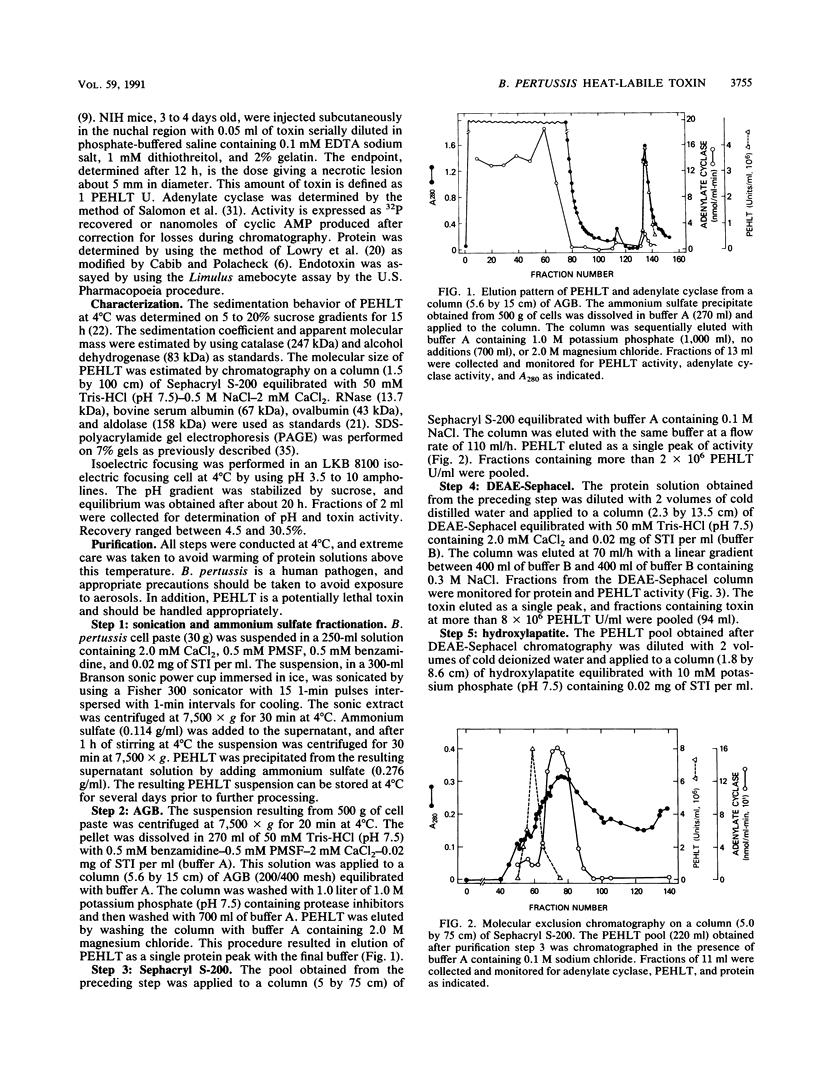
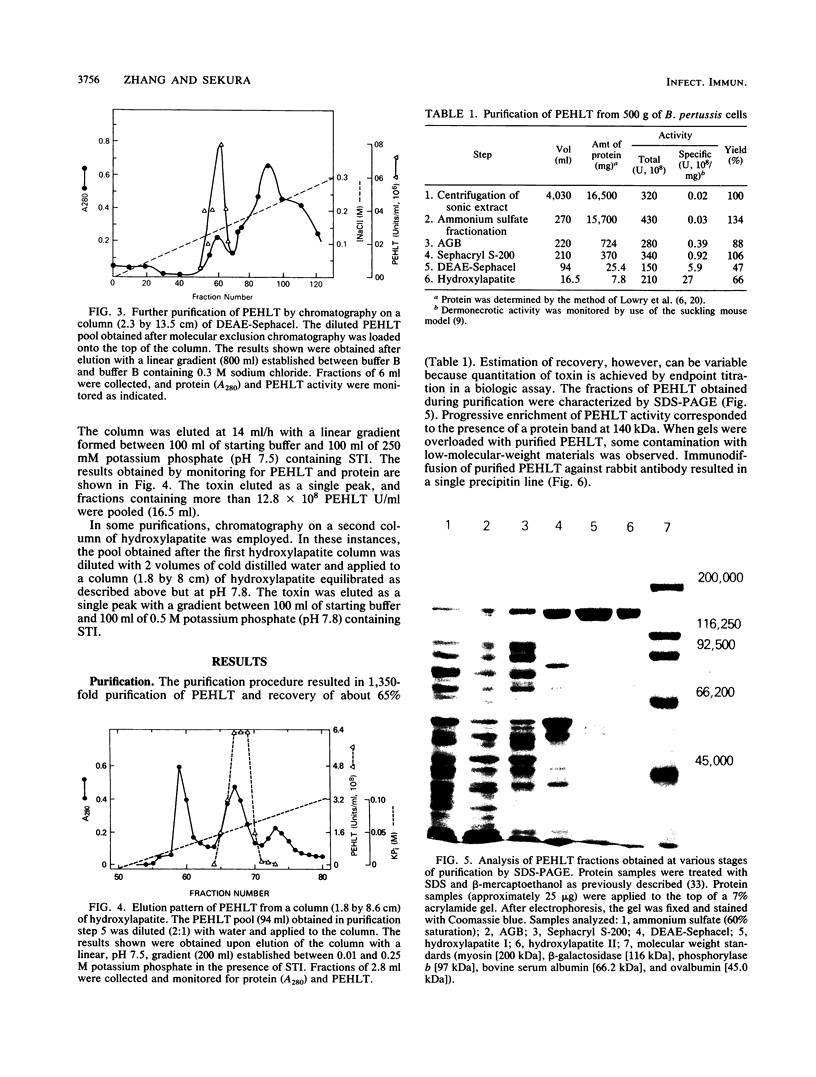
A procedure is described for purification of pertussis heat-labile toxin (PEHLT) from cells of Bordetella pertussis. The purification procedure, performed in the cold and in the presence of protease inhibitors, gives 1,350-fold purification with yields of about 60%. The toxin was shown to be a single-chain polypeptide of 140 kDa, pI 6.02. It was completely inactivated by heating at 56 degrees C for 60 min. Rabbit antiserum prepared against PEHLT neutralized the toxin and gave a single precipitin line on immunodiffusion. In immunodiffusion assays, this anti-PEHLT serum did not react with pertussis toxin, filamentous hemagglutinin, or preparations of pertussis adenylate cyclase. Purified PEHLT elicited dermonecrosis and atrophy of the spleen. PEHLT is extraordinarily active; 0.4 X 10(-12) g caused necrotic lesions in newborn mice, and with 18- to 20-g mice the 50% lethal dose was about 11 X 10(-9) g.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEA A., MUNOZ J. Antigens of Bordetella pertussis. II. Purification of heat-labile toxin. J Bacteriol. 1962 Aug;84:269–274. doi: 10.1128/jb.84.2.269-274.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Cabib E., Polacheck I. Protein assay for dilute solutions. Methods Enzymol. 1984;104:415–416. doi: 10.1016/s0076-6879(84)04108-2. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Cowell J. L., Hewlett E. L., Manclark C. R. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. doi: 10.1128/iai.25.3.896-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Amitani M., Nakase Y. Purification and characterization of heat-labile toxin from Bordetella bronchiseptica. Microbiol Immunol. 1986;30(7):659–673. doi: 10.1111/j.1348-0421.1986.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Endoh M., Nagai M., Burns D. L., Manclark C. R., Nakase Y. Effects of exogenous agents on the action of Bordetella parapertussis heat-labile toxin on guinea pig skin. Infect Immun. 1990 May;58(5):1456–1460. doi: 10.1128/iai.58.5.1456-1460.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci. 1989 Nov;14(11):459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Gordon V. M., McCaffery J. D., Sutherland W. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989 Nov 15;264(32):19379–19384. [PubMed] [Google Scholar]
- Hildebrandt J. D., Sekura R. D., Codina J., Iyengar R., Manclark C. R., Birnbaumer L. Stimulation and inhibition of adenylyl cyclases mediated by distinct regulatory proteins. Nature. 1983 Apr 21;302(5910):706–709. doi: 10.1038/302706a0. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y., Nakai T., Kume K. Purification and characterization of Bordetella bronchiseptica dermonecrotic toxin. Microb Pathog. 1989 May;6(5):361–368. doi: 10.1016/0882-4010(89)90078-8. [DOI] [PubMed] [Google Scholar]
- Kume K., Nakai T., Samejima Y., Sugimoto C. Properties of dermonecrotic toxin prepared from sonic extracts Bordetella bronchiseptica. Infect Immun. 1986 May;52(2):370–377. doi: 10.1128/iai.52.2.370-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livey I., Wardlaw A. C. Production and properties of Bordetella pertussis heat-labile toxin. J Med Microbiol. 1984 Feb;17(1):91–103. doi: 10.1099/00222615-17-1-91. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Nakase Y., Endoh M. Bordetella heat-labile toxin: further purification, characterization and mode of action. Dev Biol Stand. 1985;61:93–102. [PubMed] [Google Scholar]
- Nakase Y., Takatsu K., Tateishi M., Sekiya K., Kasuga T. Heat-labile toxin of Bordetella pertussis purified by preparative acrylamide gel electrophoresis. Jpn J Microbiol. 1969 Dec;13(4):359–366. doi: 10.1111/j.1348-0421.1969.tb00479.x. [DOI] [PubMed] [Google Scholar]
- ONOUE K., KITAGAWA M., YAMAMURA Y. CHEMICAL STUDIES ON CELLULAR COMPONENTS OF BORDETELLA PERTUSSIS. III. ISOLATION OF HIGHLY POTENT TOXIN FROM BORDETELLA PERTUSSIS. J Bacteriol. 1963 Oct;86:648–655. doi: 10.1128/jb.86.4.648-655.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENNELL R. B., THIELE E. H. Studies on the fractionation of Hemophilus pertussis extracts. J Immunol. 1951 Jun;66(6):627–633. [PubMed] [Google Scholar]
- Parton R. Effect of anti-inflammatory agents on the haemorrhagic response of mouse skin to Bordetella pertussis heat-labile toxin. J Med Microbiol. 1986 May;21(3):265–270. doi: 10.1099/00222615-21-3-265. [DOI] [PubMed] [Google Scholar]
- Parton R. Effect of prednisolone on the toxicity of Bordetella pertussis for mice. J Med Microbiol. 1985 Jun;19(3):391–400. doi: 10.1099/00222615-19-3-391. [DOI] [PubMed] [Google Scholar]
- ROBBINS K. C., PILLEMER L. Separation of pertussal toxin. Proc Soc Exp Biol Med. 1950 May;74(1):75–78. doi: 10.3181/00379727-74-17814. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]