Abstract
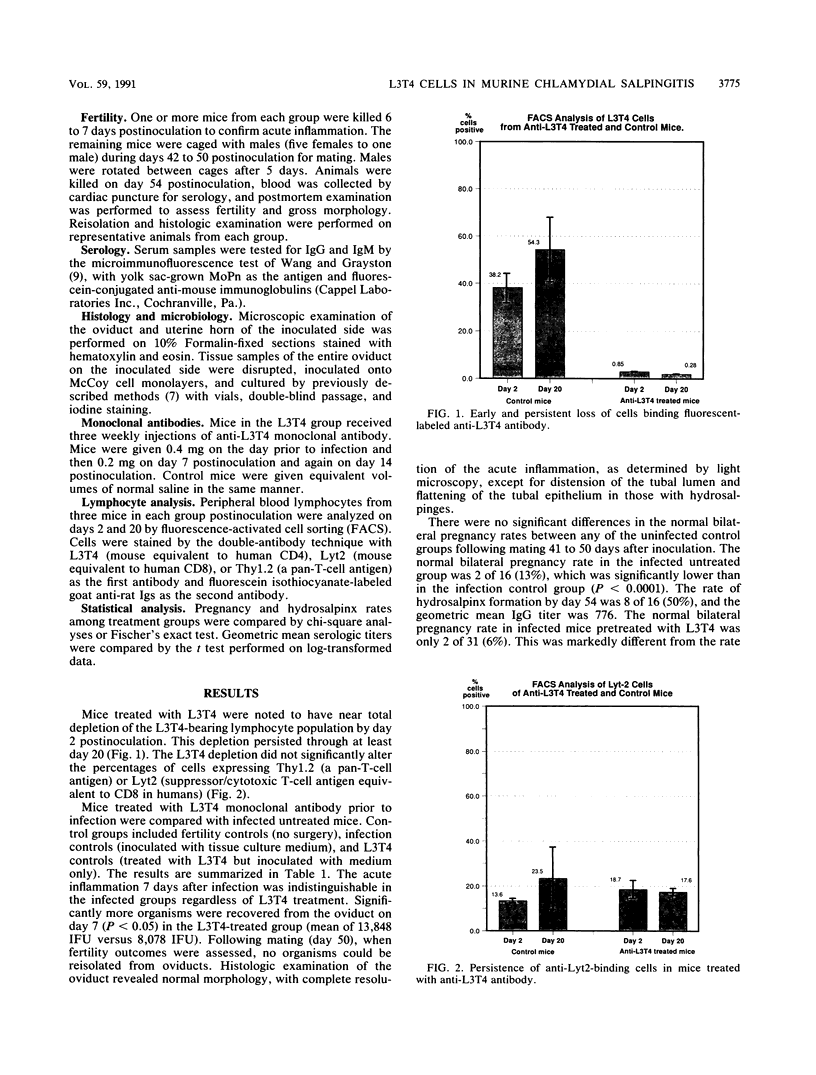
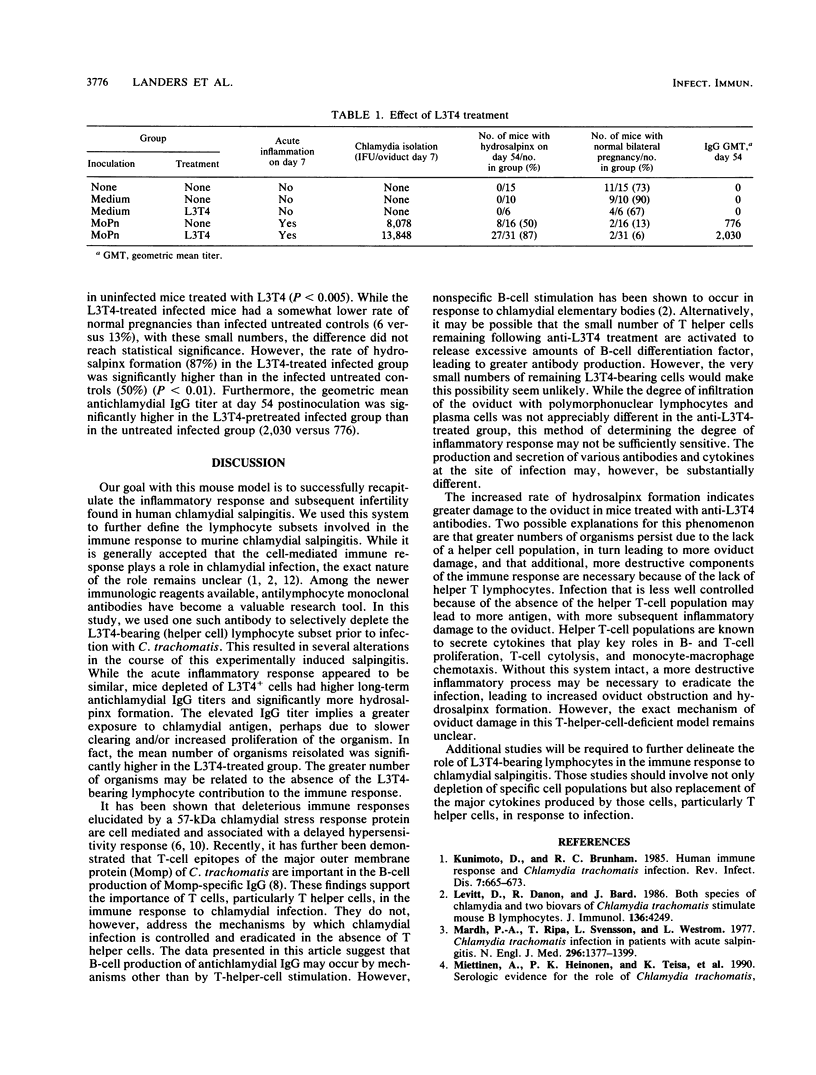
A role for both the cellular and humoral components of the immune response has been established for chlamydial infection. The significance of helper (L3T4) T cells was evaluated by using a Chlamydia trachomatis murine salpingitis model for upper genital tract chlamydial infection. Mouse oviducts were inoculated with C. trachomatis by using the mouse pneumonitis agent (MoPn) or control medium. Mice depleted of L3T4-bearing lymphocytes had significantly higher (P less than 0.05) numbers of organisms recovered at day 7 postinoculation. The rate of hydrosalpinx formation was significantly higher in the mice depleted of L3T4-bearing lymphocytes (27 of 31 [87%] ) than in the infected undepleted group (8 of 16 [50%] ) (P less than 0.01). The geometric mean antichlamydial immunoglobulin G titers at day 54 postinoculation were significantly higher in the L3T4-depleted mice (mean titer, 2,030) than in the undepleted group (mean titer, 776; P less than 0.05). The rate of fertility was lower in the L3T4-depleted group (2 of 31 [6%]) than in the infected, undepleted mice (2 of 16 [13%]), but this difference did not reach statistical significance. In conclusion, the greater persistence of organisms in the oviduct and higher rates of hydrosalpinx formation in mice depleted of L3T4-bearing cells suggests that these cells play a role in the clearing of organisms following infection and thus in reducing the degree of oviduct obstruction and damage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kunimoto D., Brunham R. C. Human immune response and Chlamydia trachomatis infection. Rev Infect Dis. 1985 Sep-Oct;7(5):665–673. doi: 10.1093/clinids/7.5.665. [DOI] [PubMed] [Google Scholar]
- Levitt D., Danen R., Bard J. Both species of chlamydia and two biovars of Chlamydia trachomatis stimulate mouse B lymphocytes. J Immunol. 1986 Jun 1;136(11):4249–4254. [PubMed] [Google Scholar]
- Miettinen A., Heinonen P. K., Teisala K., Hakkarainen K., Punnonen R. Serologic evidence for the role of Chlamydia trachomatis, Neisseria gonorrhoeae, and Mycoplasma hominis in the etiology of tubal factor infertility and ectopic pregnancy. Sex Transm Dis. 1990 Jan-Mar;17(1):10–14. [PubMed] [Google Scholar]
- Moore D. E., Spadoni L. R., Foy H. M., Wang S. P., Daling J. R., Kuo C. C., Grayston J. T., Eschenbach D. A. Increased frequency of serum antibodies to Chlamydia trachomatis in infertility due to distal tubal disease. Lancet. 1982 Sep 11;2(8298):574–577. doi: 10.1016/s0140-6736(82)90659-6. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Ripa T., Svensson L., Weström L. Chilamydia trachomatis infection in patients with acute salpingitis. N Engl J Med. 1977 Jun 16;296(24):1377–1379. doi: 10.1056/NEJM197706162962403. [DOI] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson C. E., Donegan E., Schachter J. Chlamydia trachomatis-induced salpingitis in mice. J Infect Dis. 1983 Dec;148(6):1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]



