Abstract
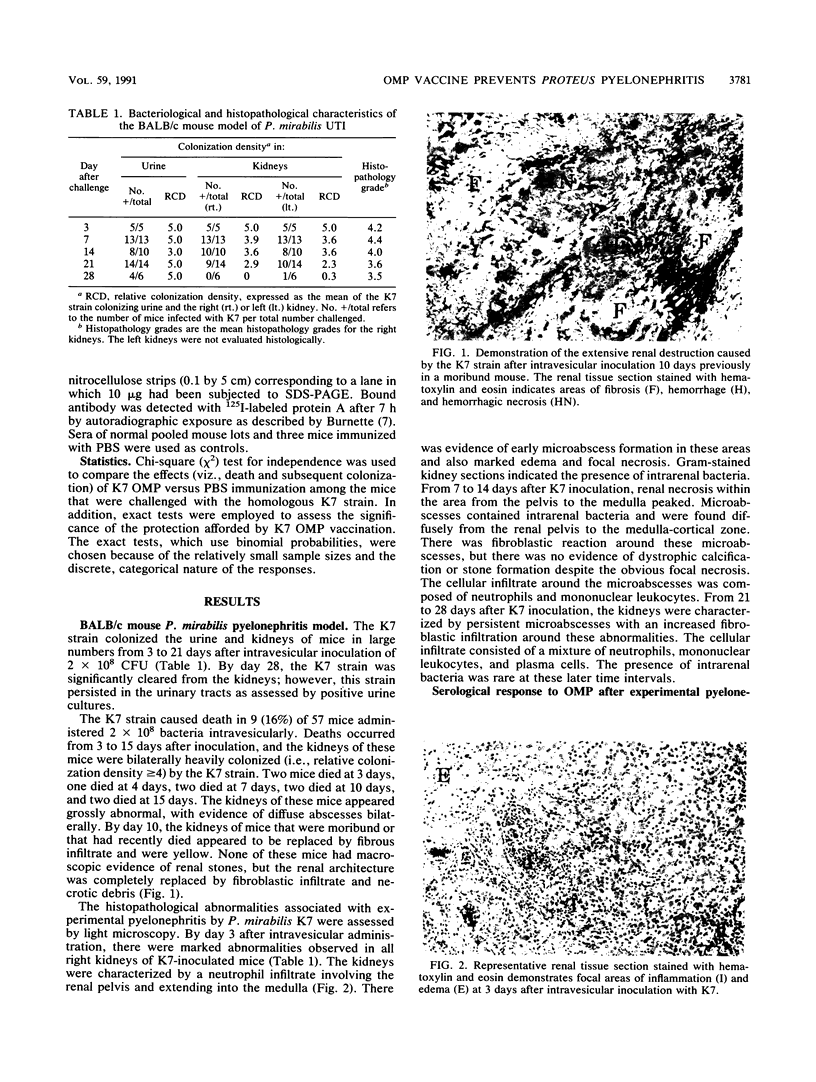
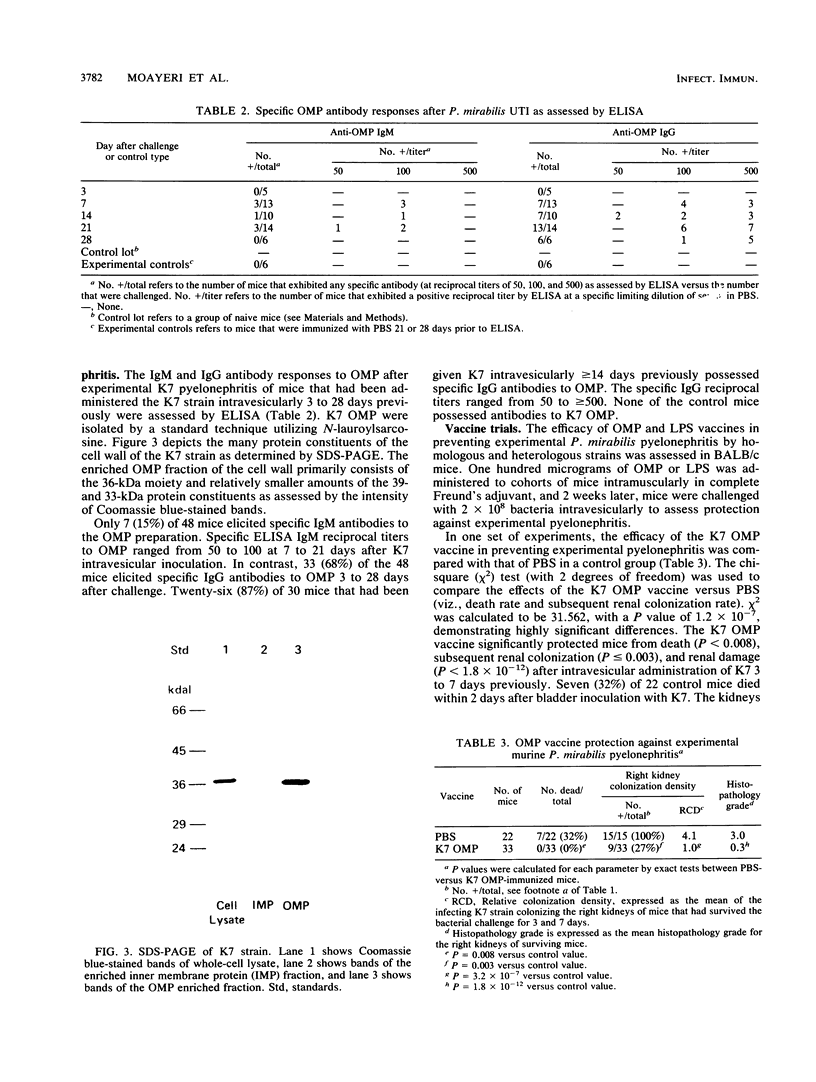
A BALB/c mouse model of nonobstructive, ascending Proteus mirabilis pyelonephritis was characterized bacteriologically, histologically, and serologically from 3 to 28 days. Intravesicular administration of 2 X 10(8) P. mirabilis K7 resulted in the septic death of 9 (16%) of 57 mice by day 15. Among the survivors, K7 colonized the kidneys in great numbers until day 21. Histological examination of the kidneys revealed acute inflammation which was characterized by neutrophil infiltration by day 3, renal necrosis by day 7, and fibroblastic infiltration by day 14 which persisted at least until day 28. The immunoglobulin G response to the outer membrane proteins (OMP) was assessed by enzyme-linked immunosorbent assay and Western blotting (immunoblotting). Anti-OMP immunoglobulin G antibodies were detected as early as day 7, and the reciprocals of their titers rose progressively up to day 28 (i.e., greater than or equal to 500). This model was also used to assess the efficacy of OMP and lipopolysaccharide (LPS) immunization in preventing renal infection. K7 OMP or LPS (100 micrograms) preparations were administered intramuscularly in Freund's complete adjuvant. After 2 weeks, mice were intravesicularly challenged with 2 X 10(8) bacteria of the homologous K7 strain or one of four heterologous strains. Compared with the saline-immunized control group and K7 LPS-immunized mice, K7 OMP recipients were protected from death when challenged by homologous or heterologous strains. In addition, K7 OMP recipients were protected (P less than 0.003) from subsequent renal infection when challenged by the K7 strain and had more rapid bacterial renal clearance when challenged by three of four heterologous strains. OMP recipients produced antibodies which bound major OMP moieties (viz., 36- to 39-kDa cell wall constituents) as assessed by Western blotting. These results support the concept that immunization with selected bacterial protein surface coat constituents can prevent uromucosal infection by interfering with colonization or renal injury.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C., Senior B. W. The adhesins and fimbriae of Proteus mirabilis strains associated with high and low affinity for the urinary tract. J Med Microbiol. 1983 Nov;16(4):427–431. doi: 10.1099/00222615-16-4-427. [DOI] [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Bub F., Bieker P., Martin H. H., Nixdorff K. Immunological characterization of two major proteins isolated from the outer membrane of Proteus mirabilis. Infect Immun. 1980 Feb;27(2):315–321. doi: 10.1128/iai.27.2.315-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Taylor P. R., Yoshikawa T. T., Guze L. B. A nosocomial outbreak of infections due to multiply resistant Proteus mirabilis: role of intestinal colonization as a major reservoir. J Infect Dis. 1979 Jun;139(6):621–627. doi: 10.1093/infdis/139.6.621. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Parker M. G., Matthews J. M., Berg R. D. Use of a purified outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine in mice. Infect Immun. 1984 Apr;44(1):49–54. doi: 10.1128/iai.44.1.49-54.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleckman R., Blagg N., Hibert D., Hall A., Crowley M., Pritchard A., Warren W. Symptomatic pyelonephritis in elderly men. J Am Geriatr Soc. 1982 Nov;30(11):690–693. doi: 10.1111/j.1532-5415.1982.tb01981.x. [DOI] [PubMed] [Google Scholar]
- Hanson L. A., Ahlstedt S., Fasth A., Jodal U., Kaijser B., Larsson P., Lindberg U., Olling S., Sohl-Akerlund A., Svanborg-Edén C. Antigens of Escherichia coli, human immune response, and the pathogenesis of urinary tract infections. J Infect Dis. 1977 Aug;136 (Suppl):S144–S149. doi: 10.1093/infdis/136.supplement.s144. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The sixth C. L. Oakley lecture. Molecular studies on the pathogenesis of gonorrhoea. J Med Microbiol. 1984 Dec;18(3):293–307. doi: 10.1099/00222615-18-3-293. [DOI] [PubMed] [Google Scholar]
- Henriksen A. Z., Maeland J. A. Immunoadsorbent-purified antibodies in the study of antigenic relatedness of outer membrane proteins of enteric bacilli. Acta Pathol Microbiol Immunol Scand B. 1986 Aug;94(4):257–263. doi: 10.1111/j.1699-0463.1986.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Major outer membrane proteins: common antigens in enterobacteriaceae species. J Gen Microbiol. 1980 Jul;119(1):123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- Hofstra H., Van Tol J. D., Dankert J. Cross-reactivity of major outer membrane proteins of Enterobacteriaceae, studied by crossed immunoelectrophoresis. J Bacteriol. 1980 Jul;143(1):328–337. doi: 10.1128/jb.143.1.328-337.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. D., Lockatell C. V., Johnson D. E., Warren J. W., Mobley H. L. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990 Apr;58(4):1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Layton G. T., Smithyman A. M. The effects of oral and combined parenteral/oral immunization against an experimental Escherichia coli urinary tract infection in mice. Clin Exp Immunol. 1983 Nov;54(2):305–312. [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren D. M. The significance of urease in proteus pyelonephritis: a bacteriological study. J Pathol Bacteriol. 1968 Jul;96(1):45–56. doi: 10.1002/path.1700960106. [DOI] [PubMed] [Google Scholar]
- MacLaren D. M. The significance of urease in proteus pyelonephritis: a histological and biochemical study. J Pathol. 1969 Jan;97(1):43–49. doi: 10.1002/path.1710970107. [DOI] [PubMed] [Google Scholar]
- Magnussen M. H., Robb S. S. Nosocomial infections in a long-term care facility. Am J Infect Control. 1980 Feb;8(1):12–17. doi: 10.1016/s0196-6553(80)80073-3. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J Infect Dis. 1990 Mar;161(3):525–530. doi: 10.1093/infdis/161.3.525. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Lifely M. R., Esdaile J. Immunity and protection of mice against Neisseria meningitidis group B by vaccination, using polysaccharide complexed with outer membrane proteins: a comparison with purified B polysaccharide. Infect Immun. 1985 Feb;47(2):527–533. doi: 10.1128/iai.47.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Griffith D. P., Yawn D., Rossen R. D. Role of urease in pyelonephritis resulting from urinary tract infection with Proteus. J Infect Dis. 1975 Feb;131(2):177–181. doi: 10.1093/infdis/131.2.177. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., Brunka J., Ujack E., Bryan L. Antibodies to major outer membrane proteins of Escherichia coli in urinary infection in the elderly. J Infect Dis. 1989 Oct;160(4):627–633. doi: 10.1093/infdis/160.4.627. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., McIntyre M., Zacharias H., MacDonell J. A. Twelve-month surveillance of infections in institutionalized elderly men. J Am Geriatr Soc. 1984 Jul;32(7):513–519. doi: 10.1111/j.1532-5415.1984.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Nicolle L. E., Ujack E., Brunka J., Bryan L. E. Immunoblot analysis of serologic response to outer membrane proteins of Escherichia coli in elderly individuals with urinary tract infections. J Clin Microbiol. 1988 Oct;26(10):2087–2091. doi: 10.1128/jcm.26.10.2087-2091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Pazin G. J., Braude A. I. Immobilizing antibodies in urine. II. Prevention of ascending spread of Proteus mirabilis. Invest Urol. 1974 Sep;12(2):129–133. [PubMed] [Google Scholar]
- Pecha B., Low D., O'Hanley P. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J Clin Invest. 1989 Jun;83(6):2102–2108. doi: 10.1172/JCI114123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerbooms P. G., Marian A., Verweij J. J., MacLaren D. M. Urinary virulence of Proteus mirabilis in two experimental mouse models. Infect Immun. 1982 Jun;36(3):1246–1248. doi: 10.1128/iai.36.3.1246-1248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Van Leeuwenhoek. 1983 Apr;49(1):1–11. doi: 10.1007/BF00457874. [DOI] [PubMed] [Google Scholar]
- Peerbooms P. G., Verweij A. M., MacLaren D. M. Uropathogenic properties of Proteus mirabilis and Proteus vulgaris. J Med Microbiol. 1985 Feb;19(1):55–60. doi: 10.1099/00222615-19-1-55. [DOI] [PubMed] [Google Scholar]
- Robledo J. A., Serrano A., Domingue G. J. Outer membrane proteins of E. coli in the host-pathogen interaction in urinary tract infection. J Urol. 1990 Feb;143(2):386–391. doi: 10.1016/s0022-5347(17)39971-8. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Hanley J., Clubb L., Fanning S. The human antibody response to uropathogenic Escherichia coli: a review. Can J Microbiol. 1988 Mar;34(3):312–318. doi: 10.1139/m88-057. [DOI] [PubMed] [Google Scholar]
- Senior B. W., Albrechtsen M., Kerr M. A. Proteus mirabilis strains of diverse type have IgA protease activity. J Med Microbiol. 1987 Sep;24(2):175–180. doi: 10.1099/00222615-24-2-175. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm W. E., Martin S. M., Bennett J. V. Epidemiology of nosocomial infection due to Gram-negative bacilli: aspects relevant to development and use of vaccines. J Infect Dis. 1977 Aug;136 (Suppl):S151–S160. doi: 10.1093/infdis/136.supplement.s151. [DOI] [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1990 Jun;58(6):1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart K. G., Welch R. A. The HpmA hemolysin is more common than HlyA among Proteus isolates. Infect Immun. 1990 Jun;58(6):1853–1860. doi: 10.1128/iai.58.6.1853-1860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Udhayakumar V., Muthukkaruppan V. R. Protective immunity induced by outer membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987 Mar;55(3):816–821. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Welch R. A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987 Sep;55(9):2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]







