Abstract
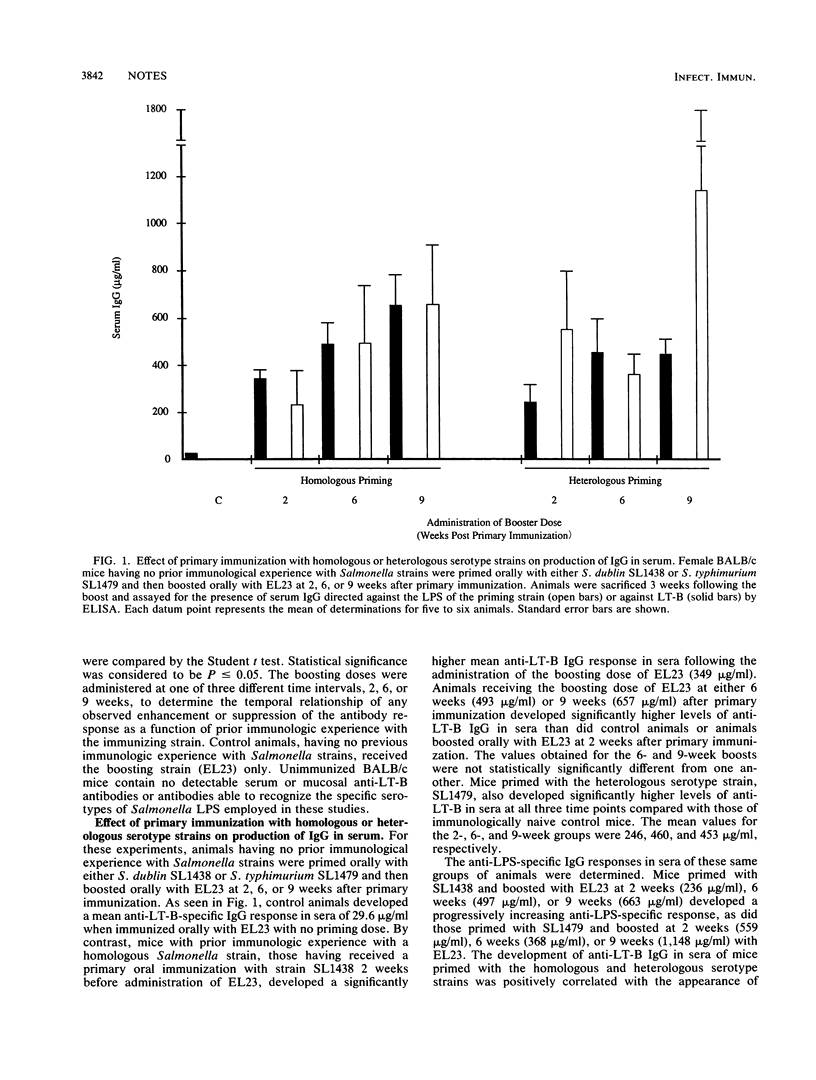
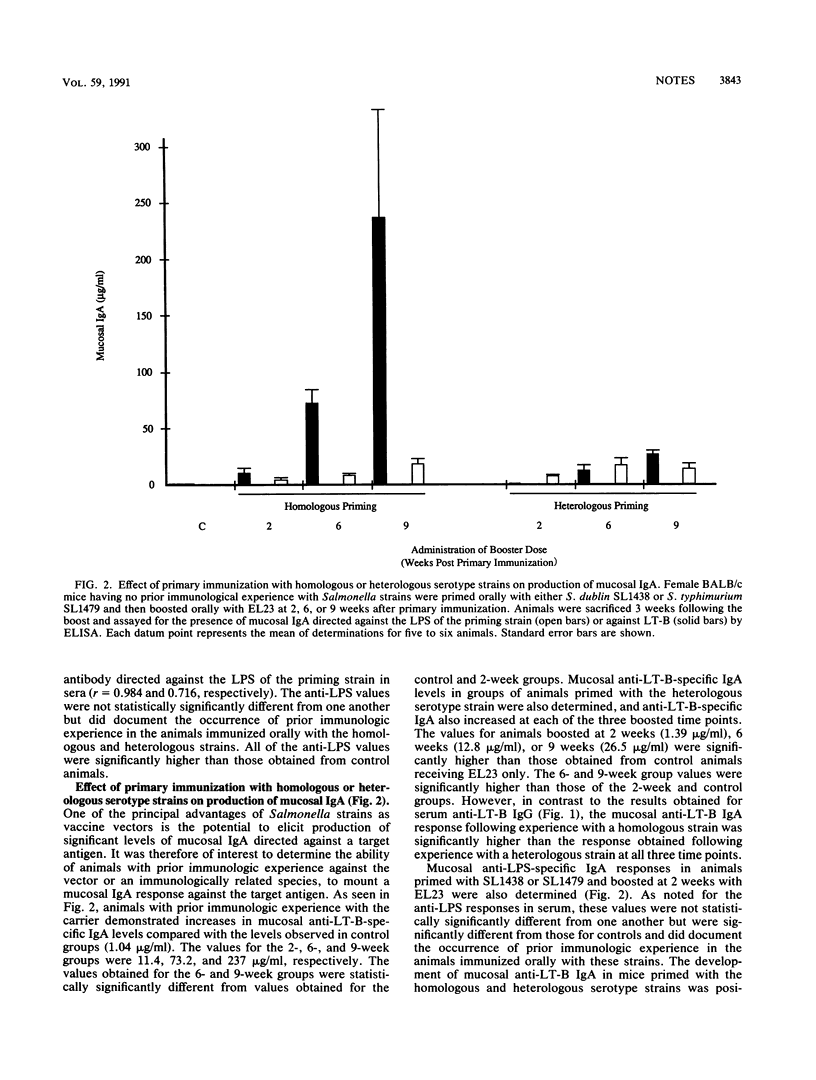
Prior immunologic experience with homologous and heterologous serotype Salmonella strains potentiated the subsequent antibody response when the same strains were used as vaccine carriers of foreign antigens. This potentiation was positively correlated with the appearance of antibody directed against the lipopolysaccharide of the carrier strain. Both serum and mucosal antibody responses against the foreign antigen increased over time. Antibody responses in sera of animals primed with either the homologous or heterologous serotype strain were not statistically significantly different, while animals primed with the homologous serotype strain developed significantly better mucosal antibody responses against the foreign antigen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Kay B. A., Khan M. U., Yunus M., Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986 Jul 19;2(8499):124–127. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Clements J. D., El-Morshidy S. Construction of a potential live oral bivalent vaccine for typhoid fever and cholera-Escherichia coli-related diarrheas. Infect Immun. 1984 Nov;46(2):564–569. doi: 10.1128/iai.46.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D. Use of attenuated mutants of Salmonella as carriers for delivery of heterologous antigens to the secretory immune system. Pathol Immunopathol Res. 1987;6(2):137–146. doi: 10.1159/000157055. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes M., Guenounou M., Ronco E., Vacheron F., Nauciel C. Impairment of lymphocyte proliferative responses and interleukin-2 production in susceptible (C57BL/6) mice infected with Salmonella typhimurium. Immunology. 1986 Jun;58(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Chatfield S., Pickard D., Bester J., O'Callaghan D., Maskell D. Construction and characterization of vaccine strains of Salmonella harboring mutations in two different aro genes. J Infect Dis. 1988 Dec;158(6):1329–1335. doi: 10.1093/infdis/158.6.1329. [DOI] [PubMed] [Google Scholar]
- Dougan G., Hormaeche C. E., Maskell D. J. Live oral Salmonella vaccines: potential use of attenuated strains as carriers of heterologous antigens to the immune system. Parasite Immunol. 1987 Mar;9(2):151–160. doi: 10.1111/j.1365-3024.1987.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Dougan G., Maskell D., Pickard D., Hormaeche C. Isolation of stable aroA mutants of Salmonella typhi Ty2: properties and preliminary characterisation in mice. Mol Gen Genet. 1987 May;207(2-3):402–405. doi: 10.1007/BF00331607. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sellwood R., Maskell D., Sweeney K., Liew F. Y., Beesley J., Hormaeche C. In vivo properties of a cloned K88 adherence antigen determinant. Infect Immun. 1986 Apr;52(1):344–347. doi: 10.1128/iai.52.1.344-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975 May;131(5):553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Hoertt B. E., Ou J., Kopecko D. J., Baron L. S., Warren R. L. Novel virulence properties of the Salmonella typhimurium virulence-associated plasmid: immune suppression and stimulation of splenomegaly. Plasmid. 1989 Jan;21(1):48–58. doi: 10.1016/0147-619x(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Delayed-type hypersensitivity and immunity to Salmonella typhimurium. Infect Immun. 1986 May;52(2):504–508. doi: 10.1128/iai.52.2.504-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Differences in delayed-type hypersensitivity responses in various mouse strains in the C3H lineage infected with Salmonella typhimurium, strain SL3235. J Immunol. 1984 Sep;133(3):1190–1196. [PubMed] [Google Scholar]
- Lee J. C., Gibson C. W., Eisenstein T. K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985 Mar;91(1):75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Ronco E., Guenet J. L. Genetic control of Salmonella typhimurium-induced depression of delayed-type hypersensitivity to sheep erythrocytes in mice. Infect Immun. 1988 Feb;56(2):310–313. doi: 10.1128/iai.56.2.310-313.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson J. A., Lindberg A. A., Hoiseth S., Stocker B. A. Salmonella typhimurium infection in calves: protection and survival of virulent challenge bacteria after immunization with live or inactivated vaccines. Infect Immun. 1983 Aug;41(2):742–750. doi: 10.1128/iai.41.2.742-750.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J. C., Ballou W. R., Baron L. S., Majarian W. R., Brey R. N., Hockmeyer W. T., Young J. F., Cryz S. J., Ou J., Lowell G. H. Oral Salmonella typhimurium vaccine expressing circumsporozoite protein protects against malaria. Science. 1988 Apr 15;240(4850):336–338. doi: 10.1126/science.3281260. [DOI] [PubMed] [Google Scholar]
- Sigwart D. F., Stocker B. A., Clements J. D. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989 Jun;57(6):1858–1861. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Stabel T. J., Mayfield J. E., Tabatabai L. B., Wannemuehler M. J. Oral immunization of mice with attenuated Salmonella typhimurium containing a recombinant plasmid which codes for production of a 31-kilodalton protein of Brucella abortus. Infect Immun. 1990 Jul;58(7):2048–2055. doi: 10.1128/iai.58.7.2048-2055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Hoiseth S. K., Smith B. P. Aromatic-dependent "Salmonella sp." as live vaccine in mice and calves. Dev Biol Stand. 1983;53:47–54. [PubMed] [Google Scholar]
- Tramont E. C., Chung R., Berman S., Keren D., Kapfer C., Formal S. B. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076-1C). J Infect Dis. 1984 Feb;149(2):133–136. doi: 10.1093/infdis/149.2.133. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Serie C., Germanier R., Lackany A., Cerisier Y., Guerin N., Sallam S., Geoffroy P., el Tantawi A. S., Guesry P. A controlled field trial of liver oral typhoid vaccine Ty21a. Bull World Health Organ. 1980;58(3):469–474. [PMC free article] [PubMed] [Google Scholar]


