Abstract
Methods for diagnosis of viral infection have progressed rapidly during the past two to three decades from animal inoculation to computer automation. Virus isolation, however, still remains the "gold standard." With the availability of antiviral agents, physicians now demand accurate laboratory diagnosis of their patients' illnesses in order to give proper treatment. Discovery of unknown viral agents still requires continued search and diligent effort.
Full text
PDF
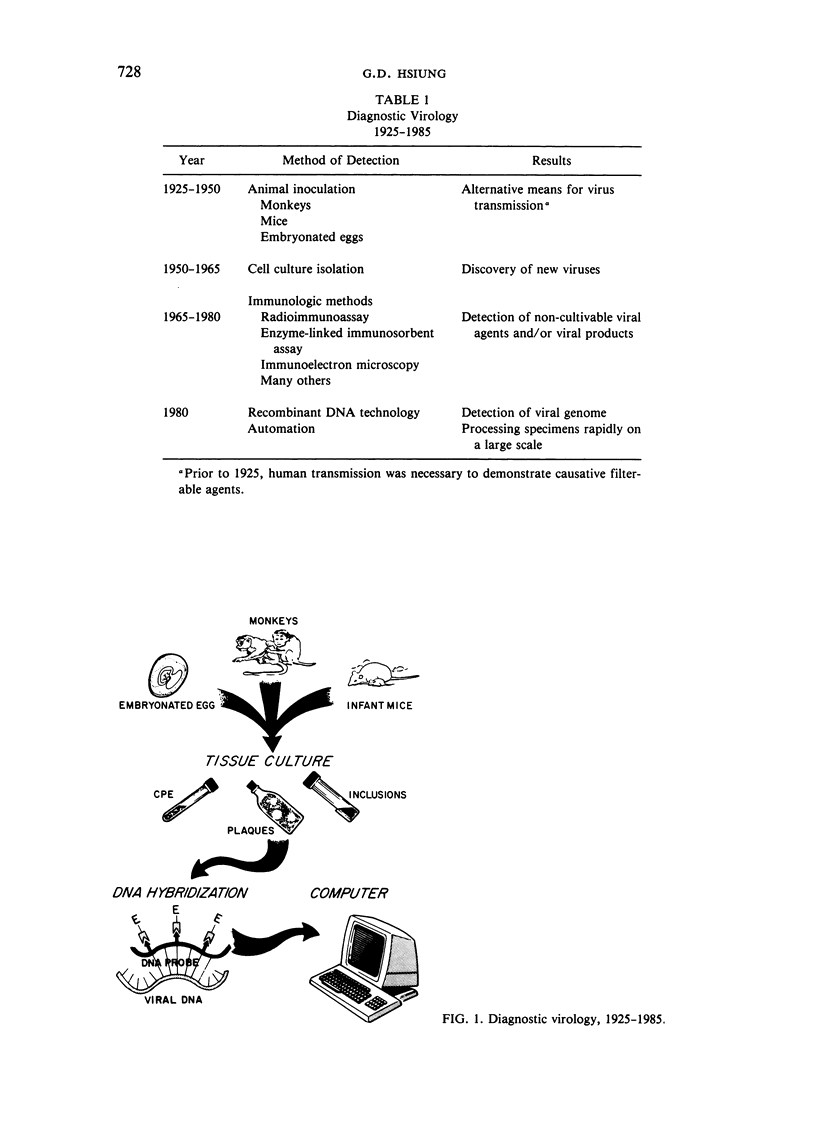
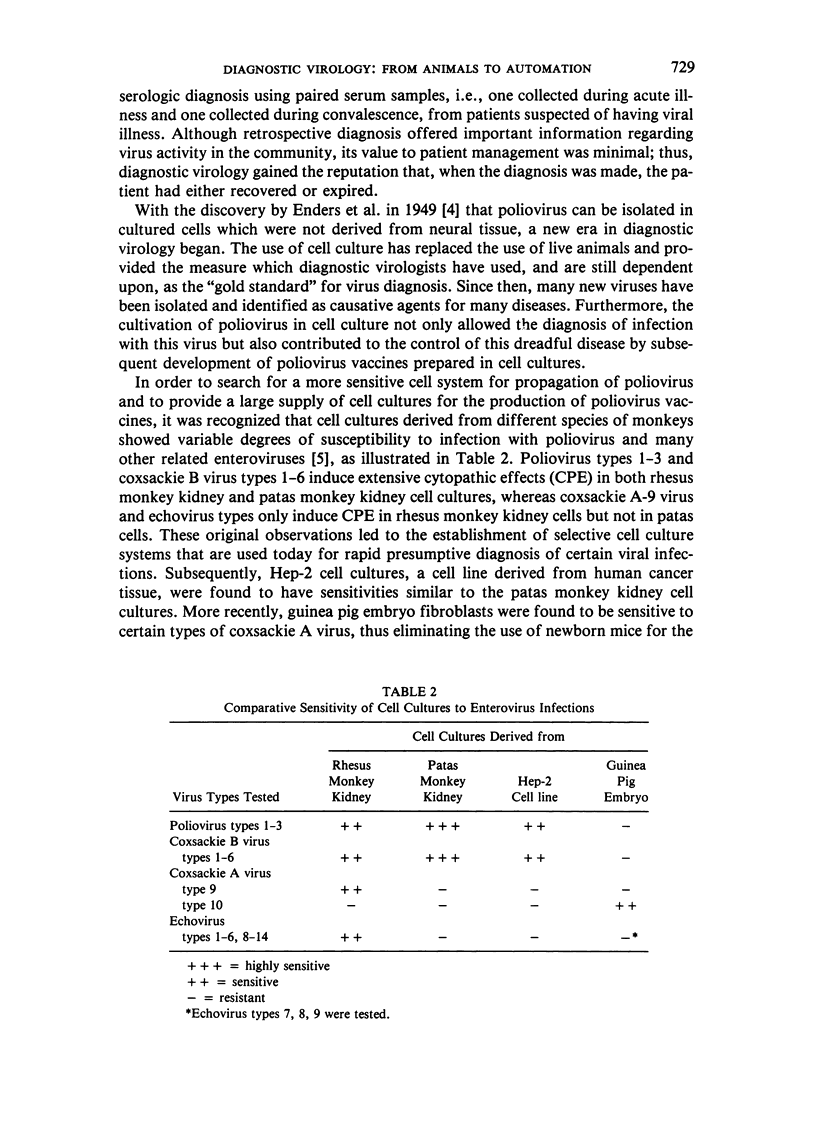

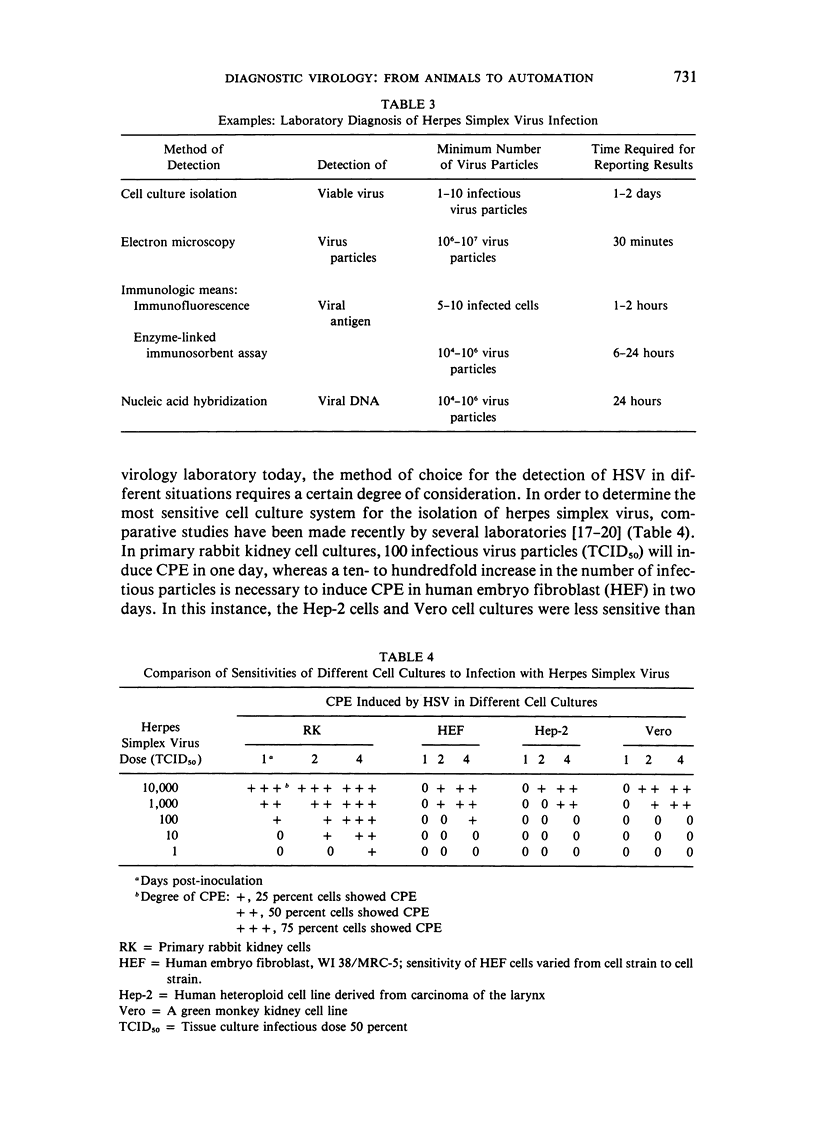


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Callihan D. R., Menegus M. A. Rapid detection of herpes simplex virus in clinical specimens with human embryonic lung fibroblast and primary rabbit kidney cell cultures. J Clin Microbiol. 1984 Apr;19(4):563–565. doi: 10.1128/jcm.19.4.563-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLDORF G. The coxsackie virus group. Ann N Y Acad Sci. 1953 Mar 31;56(3):583–586. doi: 10.1111/j.1749-6632.1953.tb30251.x. [DOI] [PubMed] [Google Scholar]
- Enders J. F., Weller T. H., Robbins F. C. Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues. Science. 1949 Jan 28;109(2822):85–87. doi: 10.1126/science.109.2822.85. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Kapikian A. Z., Purceli R. H. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973 Dec 7;182(4116):1026–1028. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H. Letter: Virus particles in gastroenteritis. Lancet. 1973 Dec 29;2(7844):1497–1497. doi: 10.1016/s0140-6736(73)92760-8. [DOI] [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Comparative susceptibility of kidney cells from different monkey species to enteric viruses (poliomyelitis, Coxsackie, and echo groups). J Immunol. 1957 Feb;78(2):137–146. [PubMed] [Google Scholar]
- HSIUNG G. D., MELNICK J. L. Morphologic characteristics of plaques produced on monkey kidney monolayer cultures by enteric viruses (poliomyelitis, Coxsackie, and echo groups. J Immunol. 1957 Feb;78(2):128–135. [PubMed] [Google Scholar]
- Hsiung G. D. Progress in clinical virology--1960 to 1980: a recollection of twenty years. Yale J Biol Med. 1980 Jan-Feb;53(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Kim H. W., Wyatt R. G., Rodriguez W. J., Ross S., Cline W. L., Parrott R. H., Chanock R. M. Reoviruslike agent in stools: association with infantile diarrhea and development of serologic tests. Science. 1974 Sep 20;185(4156):1049–1053. doi: 10.1126/science.185.4156.1049. [DOI] [PubMed] [Google Scholar]
- Landry M. L., Madore H. P., Fong C. K., Hsiung G. D. Use of guinea pig embryo cell cultures for isolation and propagation of group A coxsackieviruses. J Clin Microbiol. 1981 Mar;13(3):588–593. doi: 10.1128/jcm.13.3.588-593.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M. L., Mayo D. R., Hsiung G. D. Comparison of guinea pig embryo cells, rabbit kidney cells, and human embryonic lung fibroblast cell strains for isolation of herpes simplex virus. J Clin Microbiol. 1982 May;15(5):842–847. doi: 10.1128/jcm.15.5.842-847.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C. M., Overby L. R. Prevalence of hepatitis B virus antigen as revealed by direct radioimmune assay with 125 I-antibody. J Immunol. 1972 Oct;109(4):834–841. [PubMed] [Google Scholar]
- Moore D. F. Comparison of human fibroblast cells and primary rabbit kidney cells for isolation of herpes simplex virus. J Clin Microbiol. 1984 Apr;19(4):548–549. doi: 10.1128/jcm.19.4.548-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L. S., Namba M., Sever J. L. Comparison of standard tissue culture, tissue culture plus staining, and direct staining for detection of genital herpes simplex virus infection. J Clin Microbiol. 1984 May;19(5):631–633. doi: 10.1128/jcm.19.5.631-633.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Wong D. C., Alter H. J., Holland P. V. Microtiter solid-phase radioimmunoassay for hepatitis B antigen. Appl Microbiol. 1973 Oct;26(4):478–484. doi: 10.1128/am.26.4.478-484.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Cleveland P. H., Redfield D. C., Oxman M. N., Wahl G. M. Rapid viral diagnosis. J Infect Dis. 1984 Mar;149(3):298–310. doi: 10.1093/infdis/149.3.298. [DOI] [PubMed] [Google Scholar]
- Rubin S. J., Rogers S. Comparison of Cultureset and primary rabbit kidney cell culture for the detection of herpes simplex virus. J Clin Microbiol. 1984 Jun;19(6):920–922. doi: 10.1128/jcm.19.6.920-922.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]


