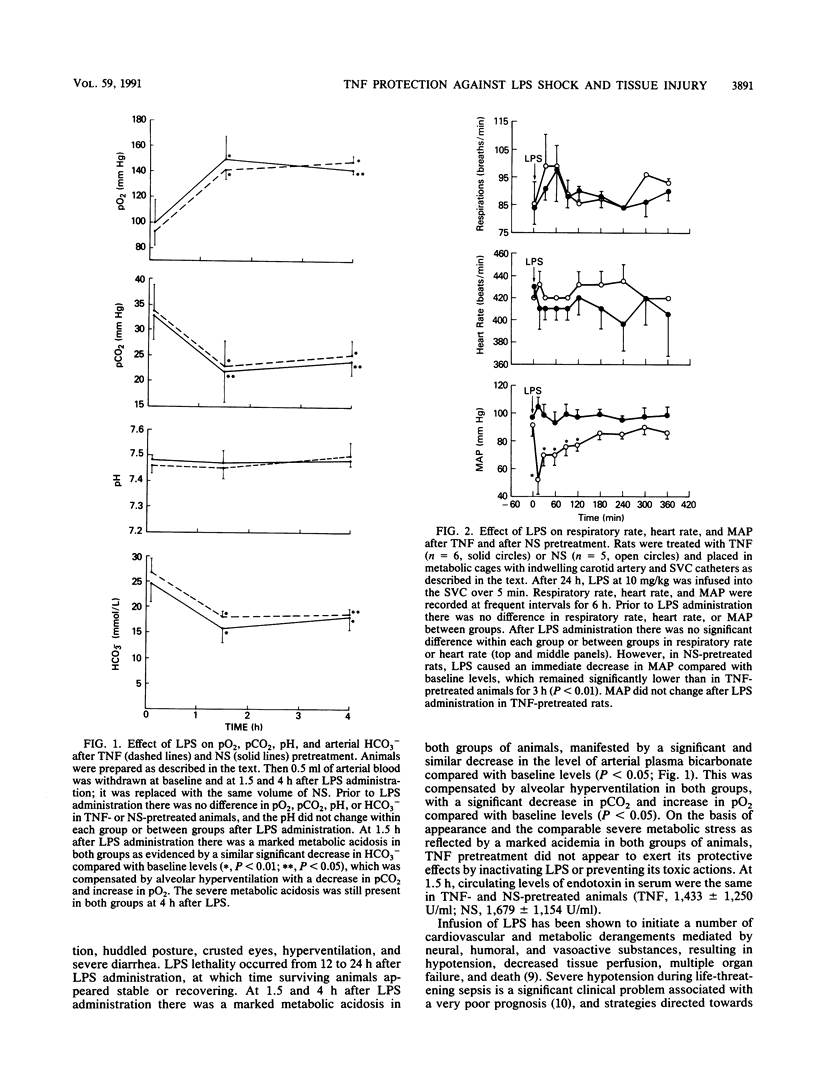
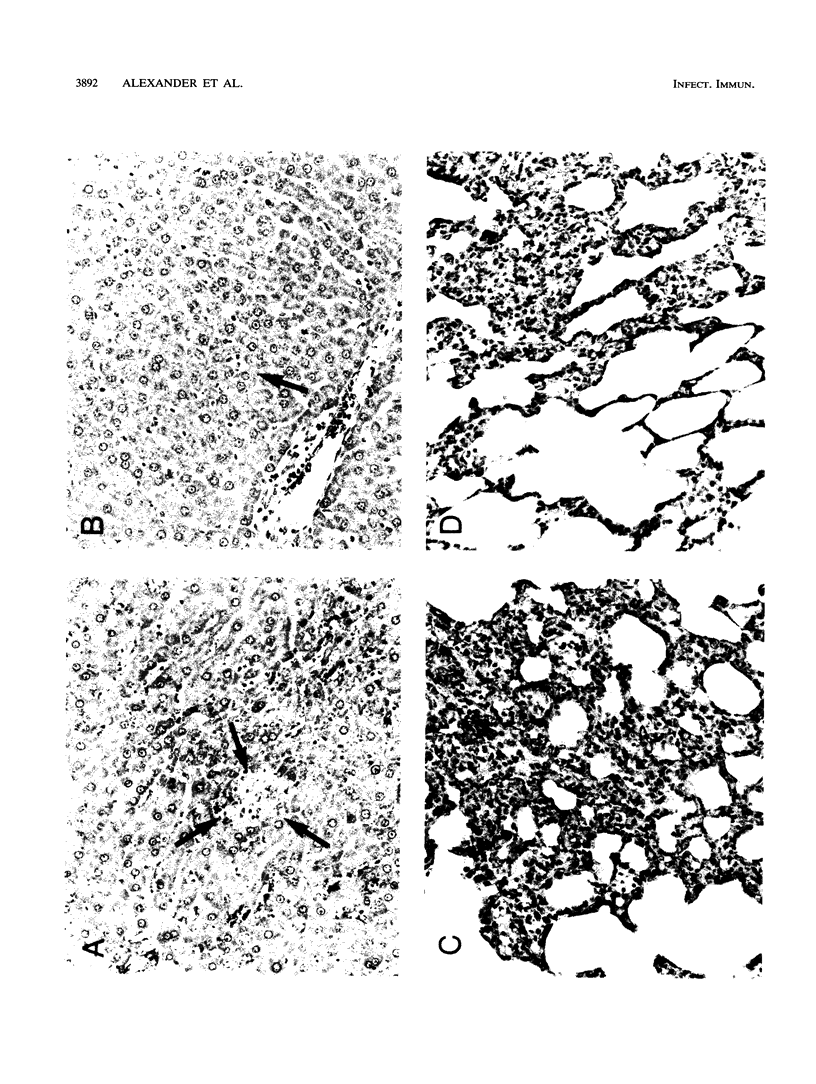
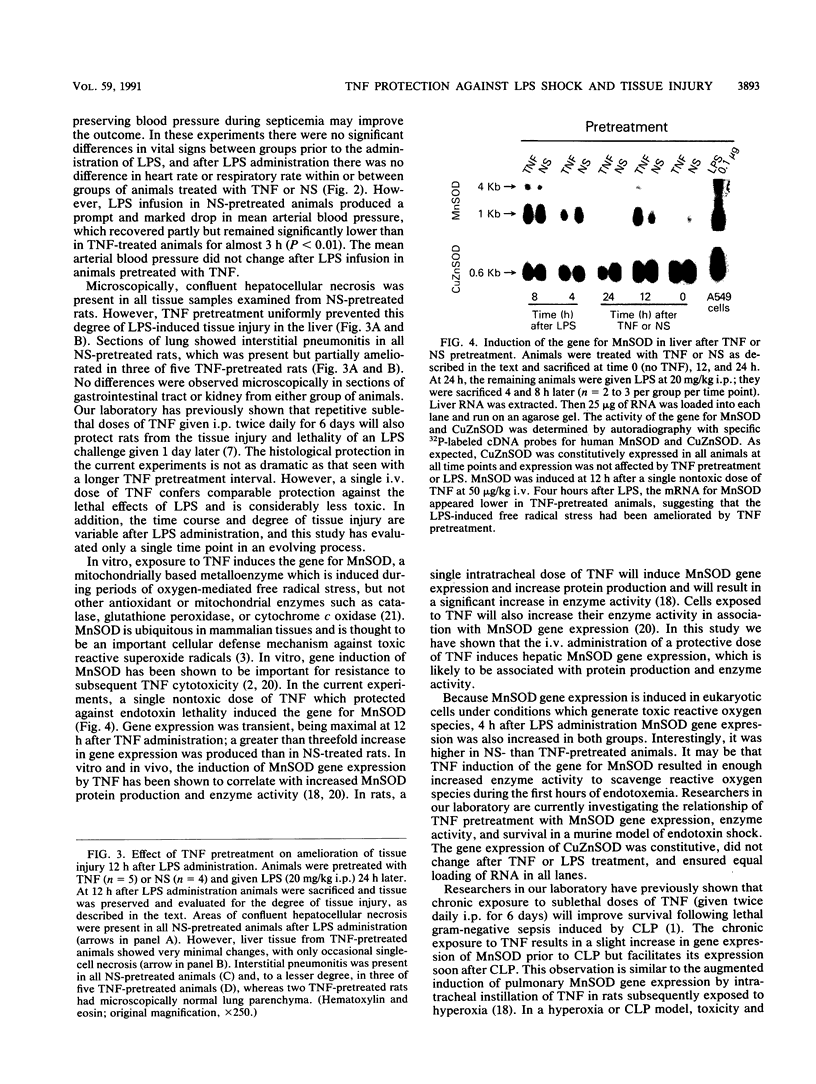
Abstract
Tumor necrosis factor (TNF), a macrophage product released in response to endotoxin and other stimuli, has been shown to be a central mediator of endotoxin or septic shock. However, its highly conserved and wide-ranging physiological effects suggest that it may also be an essential cytokine in the host defense against acute bacterial infection or sepsis. A single nontoxic dose of human recombinant TNF administered intravenously 24 h prior to a lethal infusion of Escherichia coli lipopolysaccharide (LPS) completely prevented acute LPS-induced hypotension, ameliorated tissue injury in the lungs and liver, and improved survival in male Fisher 344 rats. The protective effects of TNF were dose dependent and required a 24-h pretreatment interval. After the infusion of LPS, animals in both groups (TNF-treated animals and saline-pretreated controls) initially appeared acutely ill and had a similar severe metabolic acidosis, indicating that TNF did not inactivate or prevent the toxic effects of LPS. Twelve hours after the administration of TNF, the gene for manganous superoxide dismutase, a mitochondrial enzyme which scavenges toxic reactive oxygen species and is induced during conditions which generate a free radical stress, was expressed in liver tissue, suggesting that the induction of manganous superoxide dismutase may be an important in vivo protective mechanism against cellular injury during lethal endotoxemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. R., Sheppard B. C., Jensen J. C., Langstein H. N., Buresh C. M., Venzon D., Walker E. C., Fraker D. L., Stovroff M. C., Norton J. A. Treatment with recombinant human tumor necrosis factor-alpha protects rats against the lethality, hypotension, and hypothermia of gram-negative sepsis. J Clin Invest. 1991 Jul;88(1):34–39. doi: 10.1172/JCI115298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asoh K., Watanabe Y., Mizoguchi H., Mawatari M., Ono M., Kohno K., Kuwano M. Induction of manganese superoxide dismutase by tumor necrosis factor in human breast cancer MCF-7 cell line and its TNF-resistant variant. Biochem Biophys Res Commun. 1989 Jul 31;162(2):794–801. doi: 10.1016/0006-291x(89)92380-2. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fraker D. L., Stovroff M. C., Merino M. J., Norton J. A. Tolerance to tumor necrosis factor in rats and the relationship to endotoxin tolerance and toxicity. J Exp Med. 1988 Jul 1;168(1):95–105. doi: 10.1084/jem.168.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima H., Weil M. H., Shubin H., Cavanilles J. Hemodynamic and metabolic studies on shock associated with gram negative bacteremia. Medicine (Baltimore) 1973 Jul;52(4):287–294. doi: 10.1097/00005792-197307000-00007. [DOI] [PubMed] [Google Scholar]
- Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J Exp Med. 1990 Aug 1;172(2):599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard B. C., Fraker D. L., Norton J. A. Prevention and treatment of endotoxin and sepsis lethality with recombinant human tumor necrosis factor. Surgery. 1989 Aug;106(2):156–162. [PubMed] [Google Scholar]
- Sheppard B. C., Venzon D., Fraker D. L., Langstein H. N., Jensen J. C., Norton J. A. Prolonged survival of tumor-bearing rats with repetitive low-dose recombinant tumor necrosis factor. Cancer Res. 1990 Jul 1;50(13):3928–3933. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Santana T. A., Lee C. Y. Tracheal insufflation of tumor necrosis factor protects rats against oxygen toxicity. J Appl Physiol (1985) 1990 Mar;68(3):1211–1219. doi: 10.1152/jappl.1990.68.3.1211. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Treanor C., Shaffer J. B. Molecular basis for tumor necrosis factor-induced increase in pulmonary superoxide dismutase activities. Am J Physiol. 1990 Dec;259(6 Pt 1):L506–L512. doi: 10.1152/ajplung.1990.259.6.L506. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988 Nov 11;242(4880):941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]