Abstract
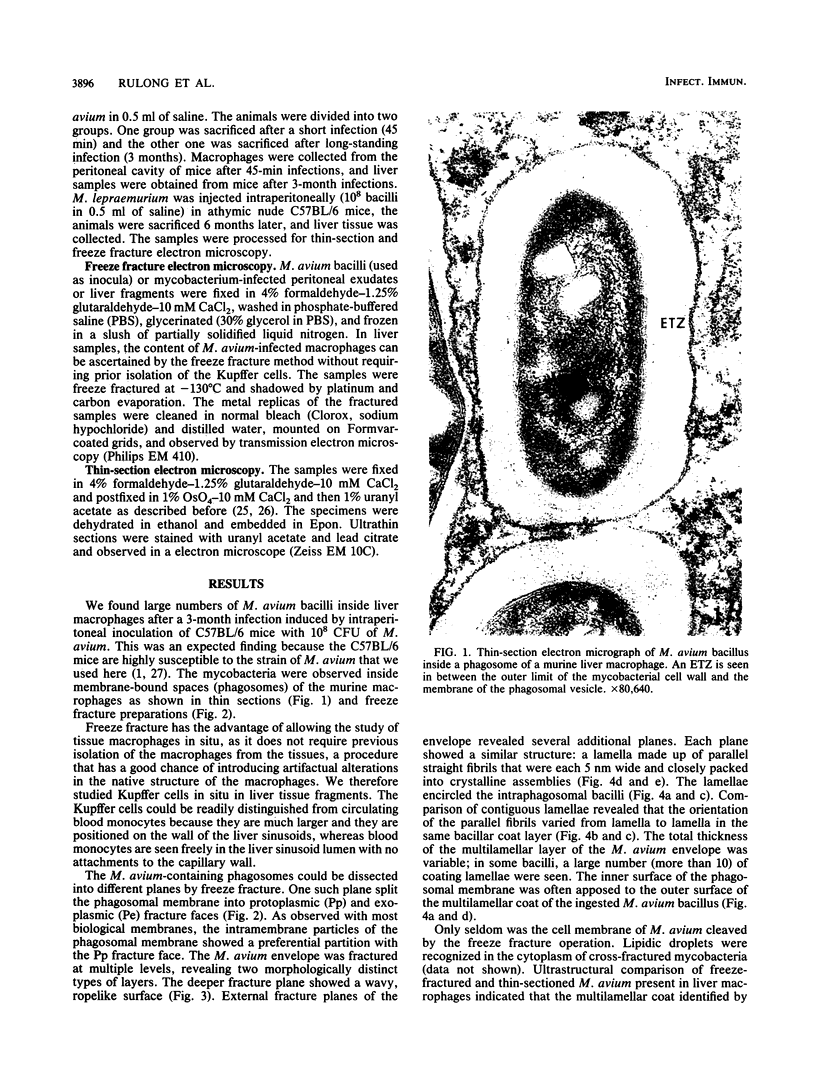
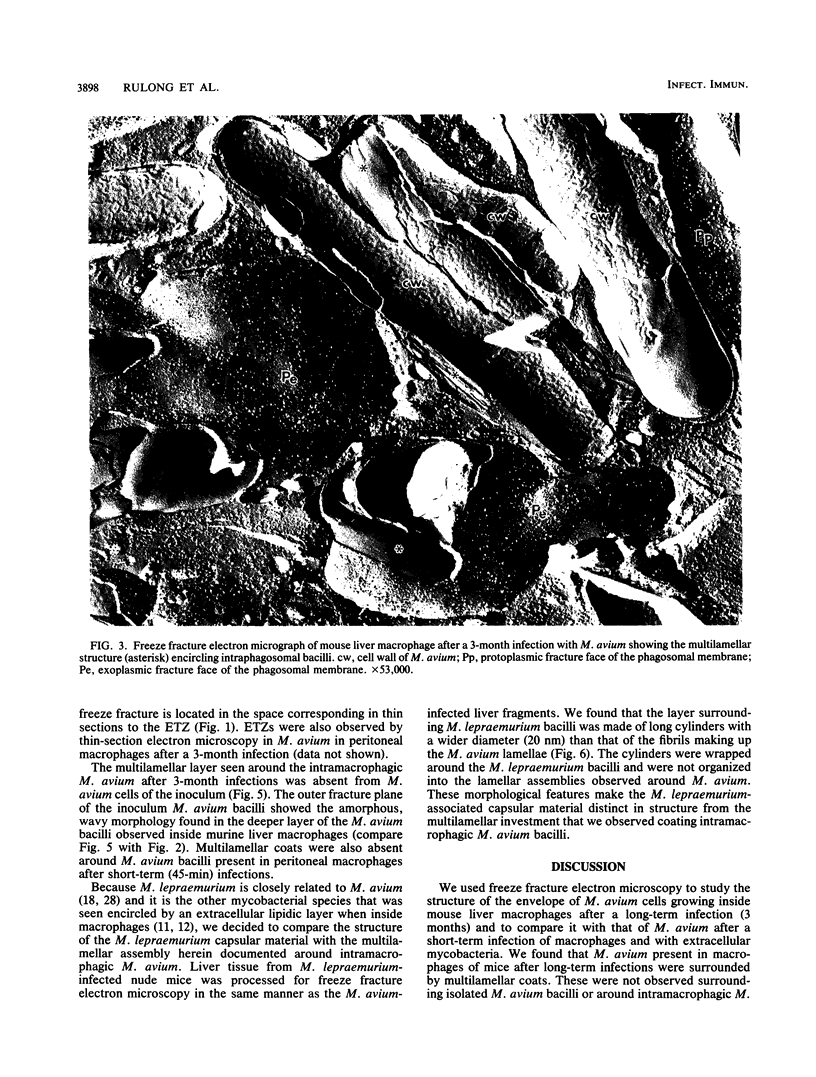
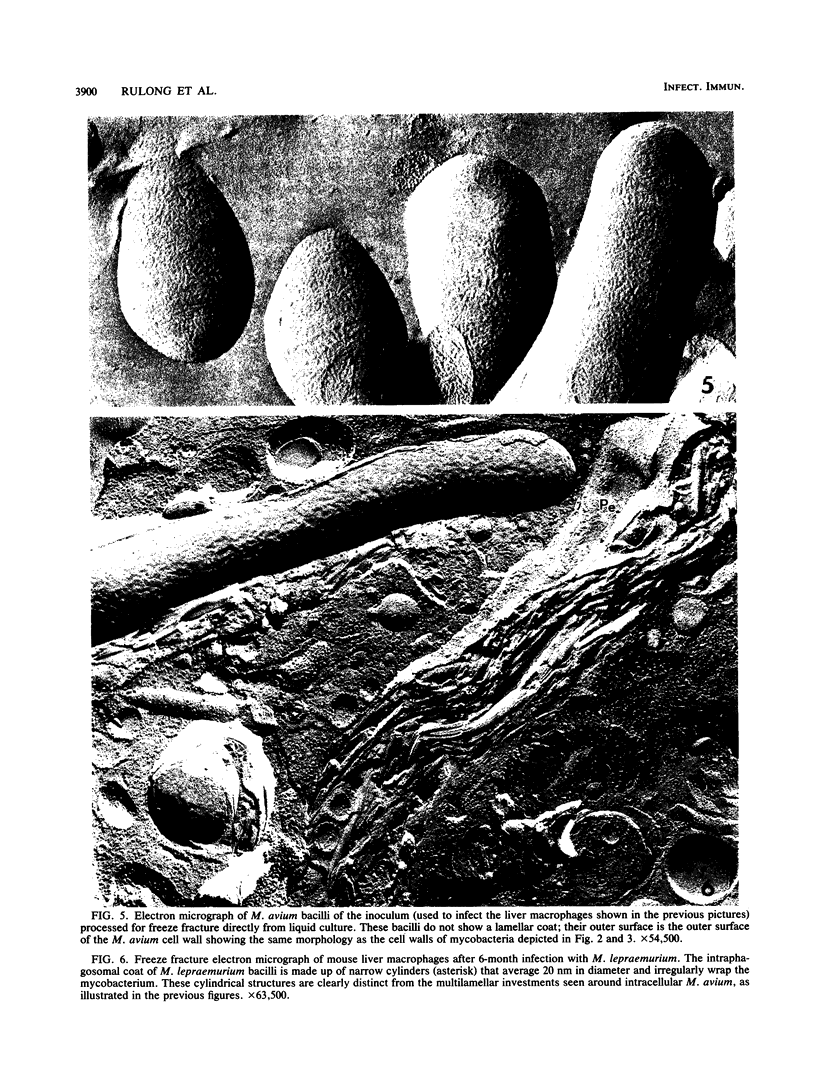
We used freeze fracture electron microscopy to study the fine structure of Mycobacterium avium inside phagosomes of murine macrophages. M. avium-susceptible C57BL/6 mice were infected with M. avium by intraperitoneal inoculation of 10(8) viable bacilli. We studied the microanatomy of the mycobacteria in 3-month infections of mice, a situation in which bacillary multiplication is extensive. In these samples, freeze fracture revealed that intraphagosomal bacilli were surrounded by a multilamellar coat that was apposed to the cell wall. In thin sections, in contrast, the area corresponding to the coat showed no substructure and was electron transparent (the so-called electron-transparent zone that has been previously reported by others). The multiple lamellae resembled an onionlike assembly that was inserted in between the mycobacterial wall outer surface and the phagosomal membrane. Each lamella of the M. avium coat was made up of parallel straight fibrils with a width of 5 nm. A variable number of lamellae, sometimes up to 10 or more elements, coated individual bacilli. The multilamellar coat was absent around both extracellular M. avium and intramacrophagic M. avium after short-term (45-min) inoculation of mice. The supramolecular organization of the M. avium lamellar coat as viewed here by freeze fracture is similar to that of purified mycoside C (P. Draper, J. Gen. Microbiol. 83:431-433, 1974; K.-S. Kim, M.R.J. Salton, and L. Barksdale, J. Bacteriol. 125:739-743, 1976), a mycobacterial component currently known as glycopeptidolipid (W.W. Barrow and P.J. Brennan, J. Bacteriol. 150:381-384, 1982). We conclude that M. avium bacilli growing in macrophages are surrounded by multilamellar capsulelike structures that contain glycopeptidolipid molecules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., Brennan P. J. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989 Jun;171(6):3465–3470. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovyagin V. L., Sabelnikov A. G. Lipid polymorphism of model and cellular membranes as revealed by electron microscopy. Electron Microsc Rev. 1989;2(1):75–115. doi: 10.1016/0892-0354(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Brennan P. J. Structure of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S420–S430. doi: 10.1093/clinids/11.supplement_2.s420. [DOI] [PubMed] [Google Scholar]
- Contreras M. A., Cheung O. T., Sanders D. E., Goldstein R. S. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis. 1988 Jan;137(1):149–152. doi: 10.1164/ajrccm/137.1.149. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Frehel C., Ryter A., Rastogi N., David H. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann Inst Pasteur Microbiol. 1986 Nov-Dec;137B(3):239–257. doi: 10.1016/s0769-2609(86)80115-6. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt E. C., Jensen D. F., Meyer P. R. Pathology of Mycobacterium avium-intracellulare infection in acquired immunodeficiency syndrome. Hum Pathol. 1987 Jul;18(7):709–714. doi: 10.1016/s0046-8177(87)80242-3. [DOI] [PubMed] [Google Scholar]
- Kwapinski J. B., Kwapinski E. H. Immunological reactions of Mycobacterium leprae and Mycobacterium lepraemurium grown in cayman. Can J Microbiol. 1973 Jun;19(6):764–766. doi: 10.1139/m73-124. [DOI] [PubMed] [Google Scholar]
- Modilevsky T., Sattler F. R., Barnes P. F. Mycobacterial disease in patients with human immunodeficiency virus infection. Arch Intern Med. 1989 Oct;149(10):2201–2205. [PubMed] [Google Scholar]
- Orme I. M., Stokes R. W., Collins F. M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986 Oct;54(1):56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R. Mycobacterium avium-intracellulare complex infections in the acquired immunodeficiency syndrome. J Electron Microsc Tech. 1988 Jan;8(1):105–113. doi: 10.1002/jemt.1060080107. [DOI] [PubMed] [Google Scholar]
- Prince D. S., Peterson D. D., Steiner R. M., Gottlieb J. E., Scott R., Israel H. L., Figueroa W. G., Fish J. E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Appelberg R., Silva M. N., Macedo P. M. In vivo killing and degradation of Mycobacterium aurum within mouse peritoneal macrophages. Infect Immun. 1987 Sep;55(9):2006–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. Ultrastructure of Mycobacterium leprae and other acid-fast bacteria as influenced by fixation conditions. Ann Microbiol (Paris) 1982 Jul-Aug;133(1):59–73. [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Stanford J. L. An immunodiffusion analysis of Mycobacterium lepraemurium Marchoux and Sorel. J Med Microbiol. 1973 Nov;6(4):435–439. doi: 10.1099/00222615-6-4-435. [DOI] [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Growth of Mycobacterium avium in activated macrophages harvested from inbred mice with differing innate susceptibilities to mycobacterial infection. Infect Immun. 1988 Sep;56(9):2250–2254. doi: 10.1128/iai.56.9.2250-2254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereletsky M. J., Barrow W. W. Postphagocytic detection of glycopeptidolipids associated with the superficial L1 layer of Mycobacterium intracellulare. Infect Immun. 1983 Sep;41(3):1312–1321. doi: 10.1128/iai.41.3.1312-1321.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba H., Crawford J. T., Ellner J. J. Pathogenicity of Mycobacterium avium for human monocytes: absence of macrophage-activating factor activity of gamma interferon. Infect Immun. 1989 Jan;57(1):239–244. doi: 10.1128/iai.57.1.239-244.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]