Abstract
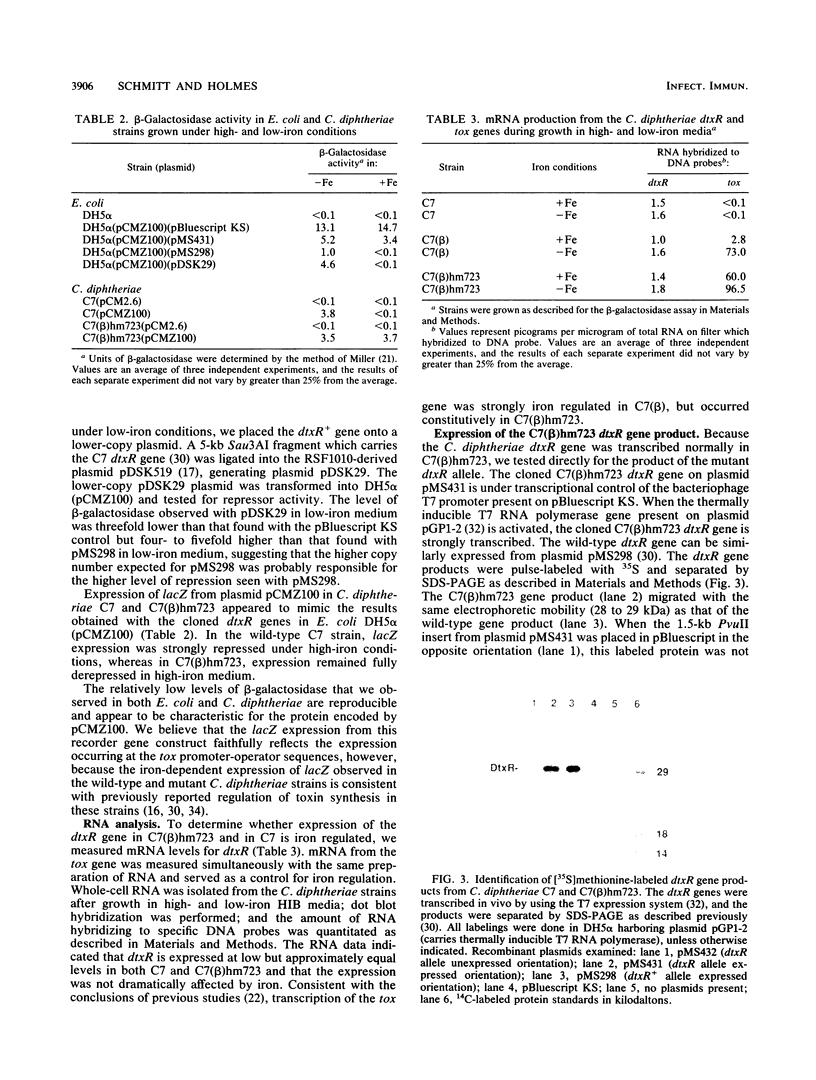
The production of diphtheria toxin and siderophore by the Corynebacterium diphtheriae regulatory mutant C7(beta)hm723 is resistant to the inhibitory effects of iron, and the mutant strain is defective for function of the regulatory gene dtxR. A 2.8-kb HindIII fragment carrying the C7(beta)hm723 dtxR allele was cloned and characterized in Escherichia coli. The restriction endonuclease maps of the 2.8-kb HindIII fragment from C7(beta)hm723 and the corresponding fragment from wild-type C. diphtheriae C7 were identical. RNA dot blot analysis with total RNA isolated from wild-type C. diphtheriae C7 and C7(beta)hm723 indicated that the dtxR gene was transcribed at very low but equivalent levels in both strains and was not regulated by iron. beta-Galactosidase synthesis from a tox-lacZ translational fusion construct in E. coli in high-iron medium was not repressed by the C7(beta)hm723dtxR allele, but was strongly repressed by the wild-type dtxR gene. The 28- to 29-kDa polypeptide expressed from the mutant dtxR allele in E. coli had the same electrophoretic mobility as the wild-type dtxR gene product in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The nucleotide sequence of the coding region and the 5' upstream region of the C7(beta)hm723 dtxR allele was determined and compared with the wild-type nucleotide sequence. The dtxR allele from C7(beta)hm723 contained a single-base change located 140 nucleotides from the 5' start of the gene, which resulted in replacement of arginine in the wild-type sequence by histidine in the mutant protein. These data demonstrate that C7(beta)hm723 expresses a mutant DtxR repressor protein that is severely defective in repressor activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J., Oza M. N., Murphy J. R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Russell L. M., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae: mutations in the bacterial genome that alter the effects of iron on toxin production. J Bacteriol. 1983 Apr;154(1):245–252. doi: 10.1128/jb.154.1.245-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Frank D. W., Iglewski B. H. Kinetics of toxA and regA mRNA accumulation in Pseudomonas aeruginosa. J Bacteriol. 1988 Oct;170(10):4477–4483. doi: 10.1128/jb.170.10.4477-4483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N. B. Conversion by corynephages and its role in the natural history of diphtheria. J Hyg (Lond) 1984 Dec;93(3):405–417. doi: 10.1017/s0022172400065001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N., Judge K. Effect of metal ions on diphtheria toxin production. Infect Immun. 1979 Dec;26(3):1065–1070. doi: 10.1128/iai.26.3.1065-1070.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii-Kanei C., Uchida T., Yoneda M. Mutants of Corynebacterium diphtheriae PW8 that produce toxin in medium with excess iron. Appl Environ Microbiol. 1981 Dec;42(6):1130–1131. doi: 10.1128/aem.42.6.1130-1131.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei C., Uchida T., Yoneda M. Isolation from corynebacterium diphtheriae C7(beta) of bacterial mutants that produce toxin in medium with excess iron. Infect Immun. 1977 Oct;18(1):203–209. doi: 10.1128/iai.18.1.203-209.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Leong D., Murphy J. R. Characterization of the diphtheria tox transcript in Corynebacterium diphtheriae and Escherichia coli. J Bacteriol. 1985 Sep;163(3):1114–1119. doi: 10.1128/jb.163.3.1114-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Michel J. L., Teng M. Evidence that the regulation of diphtheria toxin production is directed at the level of transcription. J Bacteriol. 1978 Aug;135(2):511–516. doi: 10.1128/jb.135.2.511-516.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Pappenheimer A. M., Jr, de Borms S. T. Synthesis of diphtheria tox-gene products in Escherichia coli extracts. Proc Natl Acad Sci U S A. 1974 Jan;71(1):11–15. doi: 10.1073/pnas.71.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Skiver J., McBride G. Isolation and partial characterization of a corynebacteriophage beta, tox operator constitutive-like mutant lysogen of Corynebacterium diphtheriae. J Virol. 1976 Apr;18(1):235–244. doi: 10.1128/jvi.18.1.235-244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J., Groman N., Coyle M. Plasmids in Corynebacterium diphtheriae and diphtheroids mediating erythromycin resistance. Antimicrob Agents Chemother. 1980 Nov;18(5):814–821. doi: 10.1128/aac.18.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. P., Holmes R. K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991 Jun;59(6):1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwold-Davis T. M., Groman N. B., Kao C. C. Localization of an origin of replication in Corynebacterium diphtheriae broad host range plasmid pNG2 that also functions in Escherichia coli. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):119–123. doi: 10.1016/0378-1097(90)90268-u. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Holmes R. K. Iron regulation of the cloned diphtheria toxin promoter in Escherichia coli. Infect Immun. 1988 Sep;56(9):2430–2436. doi: 10.1128/iai.56.9.2430-2436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S. P., Krafft A. E., Nootheti P., Holmes R. K. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb Pathog. 1990 Oct;9(4):267–273. doi: 10.1016/0882-4010(90)90015-i. [DOI] [PubMed] [Google Scholar]
- Welkos S. L., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae. I. Mutations in bacteriophage beta that alter the effects of iron on toxin production. J Virol. 1981 Mar;37(3):936–945. doi: 10.1128/jvi.37.3.936-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S. L., Holmes R. K. Regulation of toxinogenesis in Corynebacterium diphtheriae. II. Genetic mapping of a tox regulatory mutation in bacteriophage beta. J Virol. 1981 Mar;37(3):946–954. doi: 10.1128/jvi.37.3.946-954.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]