Abstract
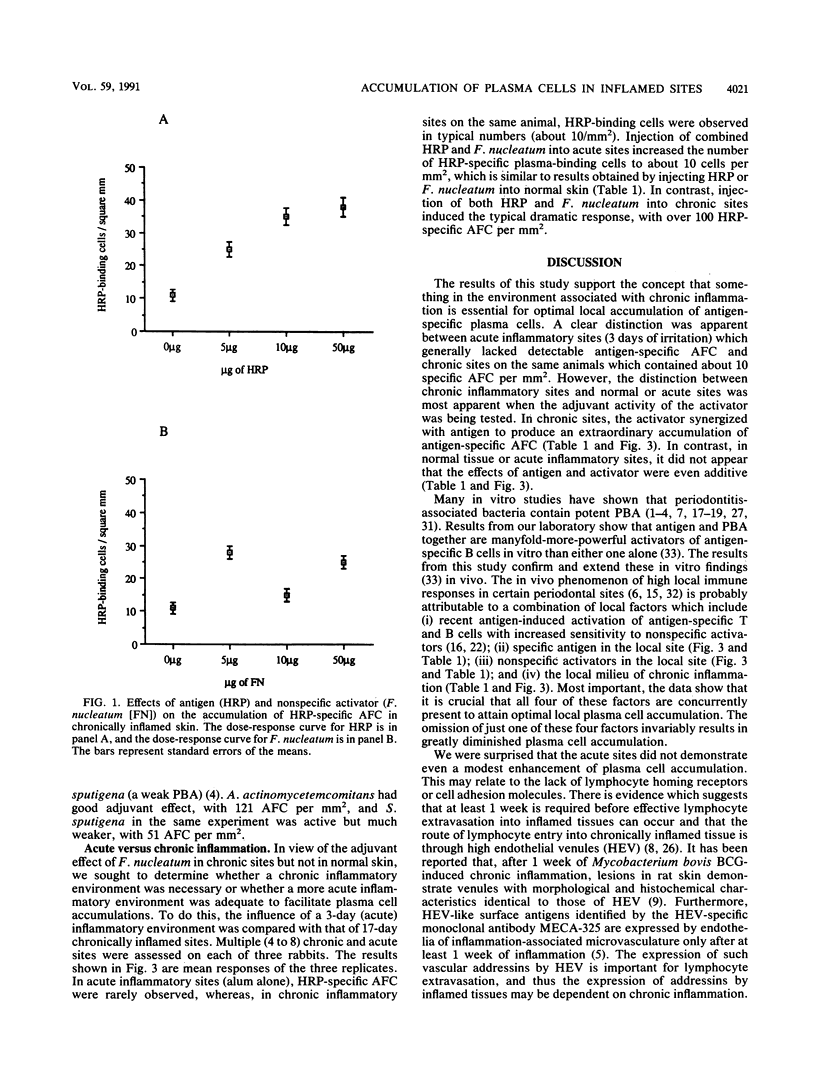
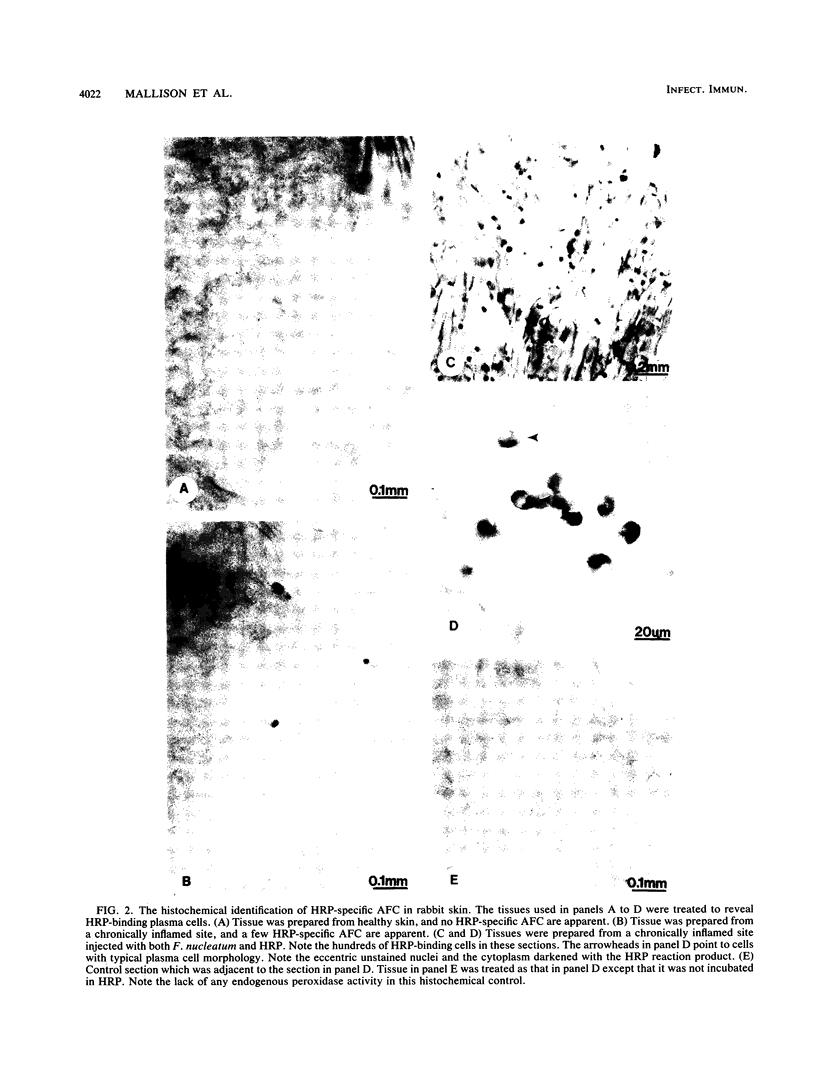
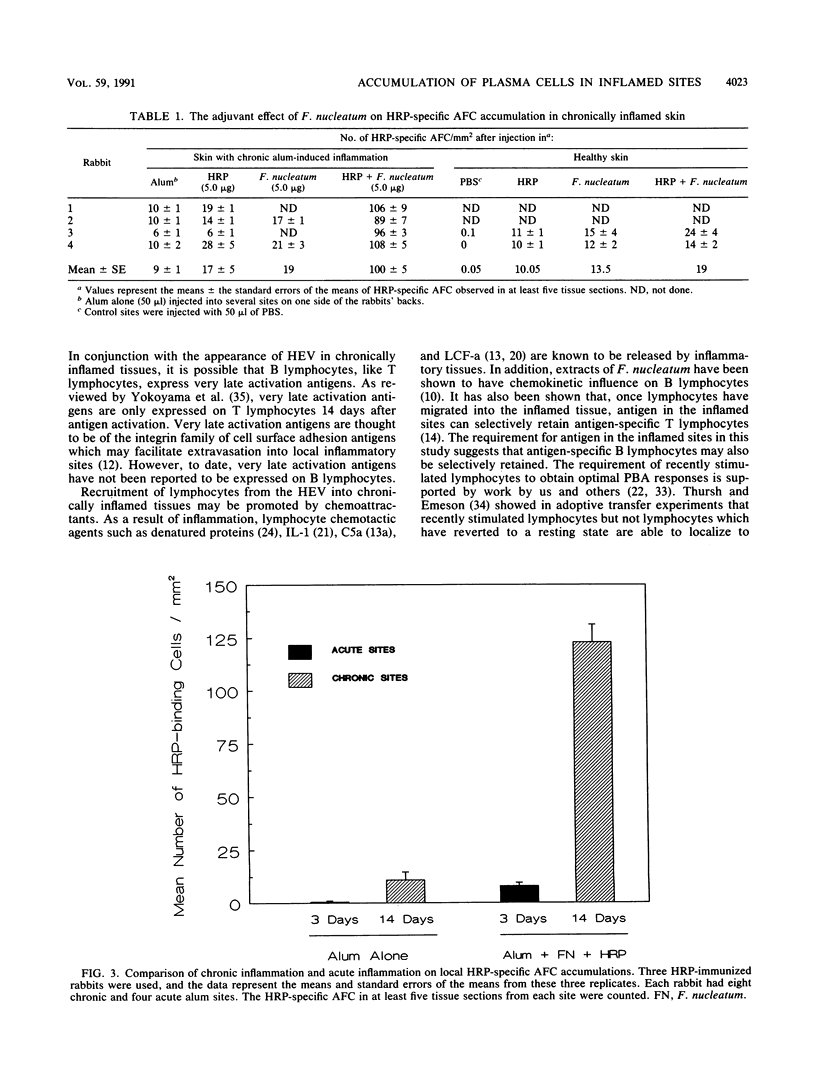
Plasma cells are common in chronically inflamed sites, including periodontal lesions. The aim of this study was to determine which factors contribute to this local accumulation of plasma cells. Specifically, we sought to evaluate the effects of specific antigen and nonspecific activators from an infectious agent associated with chronic inflammation (Fusobacterium nucleatum, an organism prominent in chronic periodontal lesions) and the effect of the chronic inflammation itself. Chronic inflammation (14 to 17 days) was induced in horseradish peroxidase (HRP)-immune rabbits by subcutaneous injection of 50 microliters of sterile alum in several sites in their backs. Controls included sites injected with saline or more acute sites examined after 3 days of alum inflammation. Sites were challenged with HRP (the antigen), sonicated F. nucleatum (the nonspecific activator), or both together to see whether F. nucleatum has an adjuvant effect. Three days after challenge, HRP-specific antibody-forming cells (AFC) were enumerated after peroxidase histochemistry. In noninflamed sites or sites with acute inflammation, virtually no HRP-specific AFC were evident. In contrast, chronic inflammation alone was sufficient to elicit a specific AFC response (congruent to 10 cells per mm2). Addition of either F. nucleatum or HRP to the chronic lesion about doubled the number of HRP-specific AFC. However, a dramatic 8- to 15-fold (80 to 150/mm2) increase was seen in chronically inflamed sites challenged with antigen and activator together. Interestingly, the activator did not have this adjuvant effect in the acute sites or in normal skin. In short, accumulation of plasma cells in inflamed sites is promoted by chronic inflammation, activators of microbial origin, and specific antigen. This milieu can be expected to develop in some periodontal lesions and could help explain why gingival crevicular fluid from some sites may contain extraordinary levels of locally produced specific antibodies for certain antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bick P. H., Carpenter A. B., Holdeman L. V., Miller G. A., Ranney R. R., Palcanis K. G., Tew J. G. Polyclonal B-cell activation induced by extracts of Gram-negative bacteria isolated from periodontally diseased sites. Infect Immun. 1981 Oct;34(1):43–49. doi: 10.1128/iai.34.1.43-49.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. B., Sully E. C., Palcanis K. G., Bick P. H. Role of monocytes in polyclonal immunoglobulin production stimulated by sonicates of periodontally associated bacteria. Infect Immun. 1983 Dec;42(3):853–862. doi: 10.1128/iai.42.3.853-862.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Donaldson S. L., Bick P. H., Moore W. E., Ranney R. R., Burmeister J. A., Tew J. G. Polyclonal B-cell activating capacities of gram-positive bacteria frequently isolated from periodontally diseased sites. J Periodontal Res. 1982 Nov;17(6):569–575. doi: 10.1111/j.1600-0765.1982.tb01178.x. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Goodson J. M. Gingival crevicular fluid antibody to oral microorganisms. I. Method of collection and analysis of antibody. J Periodontal Res. 1984 Mar;19(2):124–132. doi: 10.1111/j.1600-0765.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Engel D., Clagett J., Page R., Williams B. Mitogenic activity of Actinomyces viscosus. I. Effects on murine B and T lymphocytes, and partial characterization. J Immunol. 1977 Apr;118(4):1466–1471. [PubMed] [Google Scholar]
- Freemont A. J. A possible route for lymphocyte migration into diseased tissues. J Clin Pathol. 1983 Feb;36(2):161–166. doi: 10.1136/jcp.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Ford W. L. Functional and morphological changes in post-capillary venules in relation to lymphocytic infiltration into BCG-induced granulomata in rat skin. J Pathol. 1985 Sep;147(1):1–12. doi: 10.1002/path.1711470102. [DOI] [PubMed] [Google Scholar]
- Goto N., Akama K. Histopathological studies of reactions in mice injected with aluminum-adsorbed tetanus toxoid. Microbiol Immunol. 1982;26(12):1121–1132. doi: 10.1111/j.1348-0421.1982.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Manning M. E., Schlossman S. F., Reinherz E. L., Weiner H. L. In vivo activated T lymphocytes in the peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. N Engl J Med. 1985 May 30;312(22):1405–1411. doi: 10.1056/NEJM198505303122201. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Honda M., Shimokawa Y., Hirashima M. Chemotactic factors associated with leukocyte emigration in immune tissue injury: their separation, characterization, and functional specificity. Int Rev Cytol. 1984;89:179–250. doi: 10.1016/s0074-7696(08)61304-2. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F., Lyons C. R., O'Hara R. M., Jr, Stein-Streilein J. The antigen-induced selective recruitment of specific T lymphocytes to the lung. J Immunol. 1982 Jan;128(1):111–115. [PubMed] [Google Scholar]
- Mallison S. M., 3rd, Kaugars C., Szakal A. K., Schenkein H. A., Tew J. G. Synthesis of antibody specific for nonoral antigen in the gingiva of periodontitis patients. J Periodontal Res. 1989 May;24(3):214–216. doi: 10.1111/j.1600-0765.1989.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Mallison S. M., 3rd, Szakal A. K., Ranney R. R., Tew J. G. Antibody synthesis specific for nonoral antigens in inflamed gingiva. Infect Immun. 1988 Apr;56(4):823–830. doi: 10.1128/iai.56.4.823-830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. In vitro stimulation of immunoglobulin production from human peripheral blood lymphocytes by a soluble preparation of Actinomyces viscosus. Infect Immun. 1981 Jan;31(1):236–244. doi: 10.1128/iai.31.1.236-244.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Lopatin D. E. Polyclonal activation of human peripheral blood B lymphocytes by Fusobacterium nucleatum. Infect Immun. 1983 Jun;40(3):1104–1111. doi: 10.1128/iai.40.3.1104-1111.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Won T., Lopatin D. E. Nonspecific induction of immunoglobulin M antibodies to periodontal disease-associated microorganisms after polyclonal human B-lymphocyte activation by Fusobacterium nucleatum. Infect Immun. 1983 Sep;41(3):1038–1045. doi: 10.1128/iai.41.3.1038-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mibu Y., Shimokaway Y., Hayashi H. Lymphocyte chemotaxis in inflammation. X. Heterogeneity of chemotactic responsiveness in human T subsets towards lymphocyte chemotactic factors from delayed hypersensitivity reaction site. Immunology. 1985 Jul;55(3):473–479. [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Yu C. L., Ziff M. Lymphocyte chemotactic activity of human interleukin 1. J Immunol. 1984 Oct;133(4):2007–2011. [PubMed] [Google Scholar]
- Peters M., Fauci A. S. Selective activation of antigen-specific human B cells in recently immunized individuals by nonspecific factors in the absence of antigen. J Immunol. 1983 Feb;130(2):678–680. [PubMed] [Google Scholar]
- Russell R. J., Wilkinson P. C., Sless F., Parrott D. M. Chemotaxis of lymphoblasts. Nature. 1975 Aug 21;256(5519):646–648. doi: 10.1038/256646a0. [DOI] [PubMed] [Google Scholar]
- Schonfeld S. E., Kagan J. M. Specificity of gingival plasma cells for bacterial somatic antigens. J Periodontal Res. 1982 Jan;17(1):60–69. doi: 10.1111/j.1600-0765.1982.tb01131.x. [DOI] [PubMed] [Google Scholar]
- Smith J. B., McIntosh G. H., Morris B. The migration of cells through chronically inflamed tissues. J Pathol. 1970 Jan;100(1):21–29. doi: 10.1002/path.1711000104. [DOI] [PubMed] [Google Scholar]
- Smith S., Bick P. H., Miller G. A., Ranney R. R., Rice P. L., Lalor J. H., Tew J. G. Polyclonal B-cell activation: severe periodontal disease in young adults. Clin Immunol Immunopathol. 1980 Jul;16(3):354–366. doi: 10.1016/0090-1229(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Streefkerk J. G. Inhibition of erythrocyte pseudoperoxidase activity by treatment with hydrogen peroxide following methanol. J Histochem Cytochem. 1972 Oct;20(10):829–831. doi: 10.1177/20.10.829. [DOI] [PubMed] [Google Scholar]
- Szakal A. K., Gieringer R. L., Kosco M. H., Tew J. G. Isolated follicular dendritic cells: cytochemical antigen localization, Nomarski, SEM, and TEM morphology. J Immunol. 1985 Mar;134(3):1349–1359. [PubMed] [Google Scholar]
- Szakal A. K., Kosco M. H., Tew J. G. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. J Immunol. 1988 Jan 15;140(2):341–353. [PubMed] [Google Scholar]
- Tew J. G., Marshall D. R., Burmeister J. A., Ranney R. R. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun. 1985 Sep;49(3):487–493. doi: 10.1128/iai.49.3.487-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Thomas S. S., Ranney R. R. Fusobacterium nucleatum-mediated immunomodulation of the in vitro secondary antibody response to tetanus toxoid and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1987 Nov;22(6):506–512. doi: 10.1111/j.1600-0765.1987.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Tew J., Engel D., Mangan D. Polyclonal B-cell activation in periodontitis. J Periodontal Res. 1989 Jul;24(4):225–241. doi: 10.1111/j.1600-0765.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Thursh D. R., Emeson E. E. Selective DNA synthesis by cells specifically localizing in response to xenogeneic erythrocytes. J Exp Med. 1973 Sep 1;138(3):659–671. doi: 10.1084/jem.138.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama W. M., Maxfield S. R., Shevach E. M. Very early (VEA) and very late (VLA) activation antigens have distinct functions in T lymphocyte activation. Immunol Rev. 1989 Jun;109:153–176. doi: 10.1111/j.1600-065x.1989.tb00024.x. [DOI] [PubMed] [Google Scholar]