Abstract
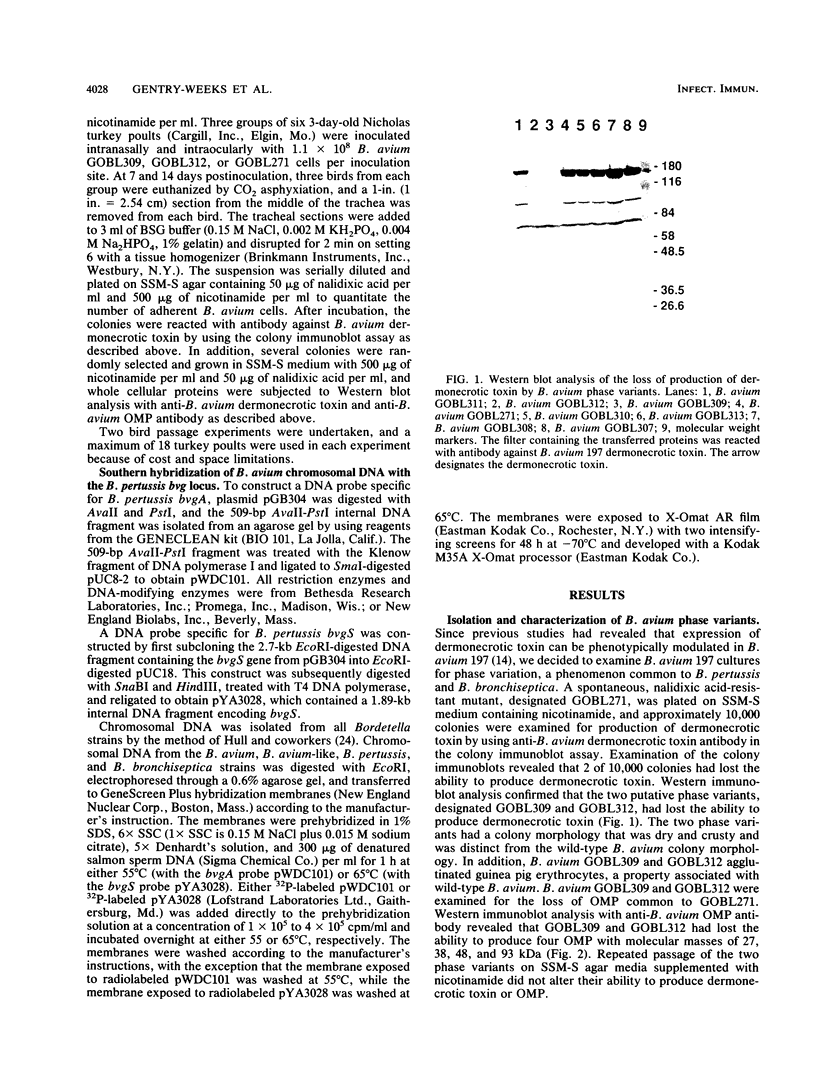
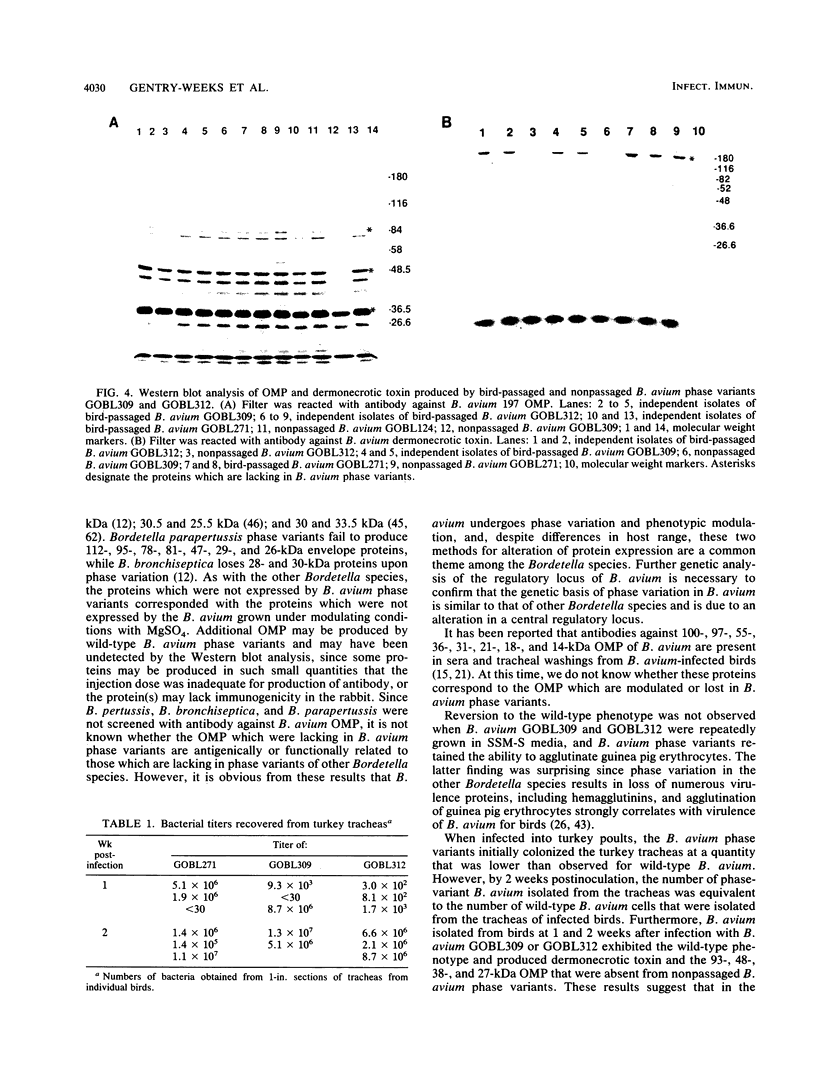
Two spontaneous phase variants of Bordetella avium were isolated at a frequency of 2 x 10(-4) by colony immunoblot assay of B. avium with antibody against B. avium dermonecrotic toxin. The two phase variants, designated GOBL309 and GOBL312, lack dermonecrotic toxin and four outer membrane proteins with molecular masses of 93, 48, 38, and 27 kDa but retain the ability to agglutinate guinea pig erythrocytes. The proteins which are not expressed by GOBL309 and GOBL312 correspond to five proteins which are phenotypically modulated in B. avium by growth in the presence of nicotinic acid or MgSO4. Growth of the phase variants in supplemented Stainer-Scholte media containing nicotinamide did not alter expression of these five proteins. Intranasal inoculation of the spontaneous phase variants into 3-day-old turkeys and reisolation of B. avium at 2 weeks postinoculation resulted in the recovery of B. avium which had the wild-type phenotype, colonized the turkey tracheas, and produced the four outer membrane proteins and dermonecrotic toxin. Hybridization of B. avium and B. avium-like chromosomal DNA with internal portions of the Bordetella pertussis virulence regulatory genes, bvgA and bvgS, revealed that B. avium and B. avium-like isolates contain 5.3- and 5.7-kb DNA fragments, respectively, which are homologous to bvgS. B. avium and B. avium-like chromosomal DNA failed to hybridize to B. pertussis bvgA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S. K., Parker C. D. Surface proteins of Bordetella pertussis: comparison of virulent and avirulent strains and effects of phenotypic modulation. Infect Immun. 1986 Nov;54(2):308–314. doi: 10.1128/iai.54.2.308-314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp L. H., Cheville N. F. Tracheal lesions in young turkeys infected with Bordetella avium. Am J Vet Res. 1984 Oct;45(10):2196–2200. [PubMed] [Google Scholar]
- Arp L. H., Fagerland J. A. Ultrastructural pathology of Bordetella avium infection in turkeys. Vet Pathol. 1987 Sep;24(5):411–418. doi: 10.1177/030098588702400508. [DOI] [PubMed] [Google Scholar]
- Arp L. H., Leyh R. D., Griffith R. W. Adherence of Bordetella avium to tracheal mucosa of turkeys: correlation with hemagglutination. Am J Vet Res. 1988 May;49(5):693–696. [PubMed] [Google Scholar]
- Berkhoff H. A., Riddle G. D. Differentiation of Alcaligenes-like bacteria of avian origin and comparison with Alcaligenes spp. reference strains. J Clin Microbiol. 1984 Apr;19(4):477–481. doi: 10.1128/jcm.19.4.477-481.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniel E., Mazigh D., Mollaret H. H. Expression of iron-regulated proteins in Yersinia species and their relation to virulence. Infect Immun. 1987 Jan;55(1):277–280. doi: 10.1128/iai.55.1.277-280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter E. J., Preston N. W. Pulmonary pertussis infection in the mouse: no solution to an old problem. J Infect. 1981 Mar;3(1):86–88. doi: 10.1016/s0163-4453(81)92461-0. [DOI] [PubMed] [Google Scholar]
- DiRita V. J., Mekalanos J. J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981 Apr;143(4):562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- Field L. H., Headley V. L., Payne S. M., Berry L. J. Influence of iron on growth, morphology, outer membrane protein composition, and synthesis of siderophores in Campylobacter jejuni. Infect Immun. 1986 Oct;54(1):126–132. doi: 10.1128/iai.54.1.126-132.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Cookson B. T., Goldman W. E., Rimler R. B., Porter S. B., Curtiss R., 3rd Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect Immun. 1988 Jul;56(7):1698–1707. doi: 10.1128/iai.56.7.1698-1707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. G., Roberts J. F., Dillman R. C., Simmons D. G. Pathogenesis of change in the upper respiratory tracts of turkeys experimentally infected with an Alcaligenes faecalis isolate. Infect Immun. 1983 Oct;42(1):350–355. doi: 10.1128/iai.42.1.350-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Families of bacterial signal-transducing proteins. Mol Microbiol. 1989 Nov;3(11):1661–1667. doi: 10.1111/j.1365-2958.1989.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hellwig D. H., Arp L. H. Identification of Bordetella avium antigens recognized after experimental inoculation in turkeys. Am J Vet Res. 1990 Aug;51(8):1188–1191. [PubMed] [Google Scholar]
- Hinz K. H., Glünder G., Lüders H. Acute respiratory disease in turkey poults caused by Bordetella bronchiseptica-like bacteria. Vet Rec. 1978 Sep 16;103(12):262–263. doi: 10.1136/vr.103.12.262. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idigbe E. O., Parton R., Wardlaw A. C. Rapidity of antigenic modulation of Bordetella pertussis in modified Hornibrook medium. J Med Microbiol. 1981 Nov;14(4):409–418. doi: 10.1099/00222615-14-4-409. [DOI] [PubMed] [Google Scholar]
- Jackwood M. W., Saif Y. M., Moorhead P. D., Dearth R. N. Further characterization of the agent causing coryza in turkeys. Avian Dis. 1985 Jul-Sep;29(3):690–705. [PubMed] [Google Scholar]
- Keevil C. W., Major N. C., Davies D. B., Robinson A. Physiology and virulence determinants of Neisseria gonorrhoeae grown in glucose-, oxygen- or cystine-limited continuous culture. J Gen Microbiol. 1986 Dec;132(12):3289–3302. doi: 10.1099/00221287-132-12-3289. [DOI] [PubMed] [Google Scholar]
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Blackmon B., Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984 Jan;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T. Regulation of virulence genes in Shigella. Mol Biol Med. 1989 Oct;6(5):425–432. [PubMed] [Google Scholar]
- McPheat W. L., Wardlaw A. C., Novotny P. Modulation of Bordetella pertussis by nicotinic acid. Infect Immun. 1983 Aug;41(2):516–522. doi: 10.1128/iai.41.2.516-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Affinity filters, a new approach to the isolation of tox mutants of Vibrio cholerae. Proc Natl Acad Sci U S A. 1978 Feb;75(2):941–945. doi: 10.1073/pnas.75.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Melton A. R., Weiss A. A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J Bacteriol. 1989 Nov;171(11):6206–6212. doi: 10.1128/jb.171.11.6206-6212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Monack D. M., Arico B., Rappuoli R., Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989 Dec;3(12):1719–1728. doi: 10.1111/j.1365-2958.1989.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Nagano H., Nakai T., Horiguchi Y., Kume K. Isolation and characterization of mutant strains of Bordetella bronchiseptica lacking dermonecrotic toxin-producing ability. J Clin Microbiol. 1988 Oct;26(10):1983–1987. doi: 10.1128/jcm.26.10.1983-1987.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy B., Grumbles L. C., Terry R. J., Millar D. L., Hall C. F. Bacterial coryza in turkeys in Texas. Poult Sci. 1981 Jan;60(1):107–113. doi: 10.3382/ps.0600107. [DOI] [PubMed] [Google Scholar]
- Parton R., Wardlaw A. C. Cell-envelope proteins of Bordetella pertussis. J Med Microbiol. 1975 Feb;8(1):47–57. doi: 10.1099/00222615-8-1-47. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S., Schrumpf M. E. Phenotypic variation and modulation in Bordetella bronchiseptica. Infect Immun. 1984 Jun;44(3):681–687. doi: 10.1128/iai.44.3.681-687.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Munson R. S., Jr, Ross R. F. Outer membrane protein profiles of Haemophilus pleuropneumoniae. Infect Immun. 1986 May;52(2):414–420. doi: 10.1128/iai.52.2.414-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler R. B., Rhoades K. R. Turkey coryza: selected tests for detection and neutralization of Bordetella avium heat-labile toxin. Avian Dis. 1986 Oct-Dec;30(4):808–812. [PubMed] [Google Scholar]
- Robinson A., Hawkins D. C. Structure and biological properties of solubilized envelope proteins of Bordetella pertussis. Infect Immun. 1983 Feb;39(2):590–598. doi: 10.1128/iai.39.2.590-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy C. R., Miller J. F., Falkow S. The bvgA gene of Bordetella pertussis encodes a transcriptional activator required for coordinate regulation of several virulence genes. J Bacteriol. 1989 Nov;171(11):6338–6344. doi: 10.1128/jb.171.11.6338-6344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N. K., Das G., Balganesh T. S., Dey S. N., Ghosh R. K., Das J. Enterotoxin production, DNA repair and alkaline phosphatase of Vibrio cholerae before and after animal passage. J Gen Microbiol. 1982 Sep;128(9):1927–1932. doi: 10.1099/00221287-128-9-1927. [DOI] [PubMed] [Google Scholar]
- Saif Y. M., Moorhead P. D., Dearth R. N., Jackwood D. J. Observations on Alcaligenes faecalis infection in turkeys. Avian Dis. 1980 Jul-Sep;24(3):665–684. [PubMed] [Google Scholar]
- Saif Y. M., Moorhead P. D., Whitmoyer R. E. Scanning electron microscopy of tracheas from turkey poults infected with Alcaligenes faecalis. Avian Dis. 1981 Jul-Sep;25(3):730–735. [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricó B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D. R., Parker C. D. Effect of pyridines on phenotypic properties of Bordetella pertussis. Infect Immun. 1982 Nov;38(2):548–553. doi: 10.1128/iai.38.2.548-553.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. G., Rose L. P., Brogden K. A., Rimler R. B. Partial characterization of the hemagglutinin of Alcaligenes faecalis. Avian Dis. 1984 Jul-Sep;28(3):700–709. [PubMed] [Google Scholar]
- Slavik M. F., Skeeles J. K., Beasley J. N., Harris G. C., Roblee P., Hellwig D. Effect of humidity on infection of turkeys with Alcaligenes faecalis. Avian Dis. 1981 Oct-Dec;25(4):936–942. [PubMed] [Google Scholar]
- Stibitz S., Aaronson W., Monack D., Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989 Mar 16;338(6212):266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw A. C., Parton R., Hooker M. J. Loss of protective antigen, histamine-sensitising factor and envelope polypeptides in cultural variants of Bordetella pertussis. J Med Microbiol. 1976 Feb;9(1):89–100. doi: 10.1099/00222615-9-1-89. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Chamness T. W., Goguen J. D. Temperature-controlled plasmid regulon associated with low calcium response in Yersinia pestis. J Bacteriol. 1986 Feb;165(2):443–447. doi: 10.1128/jb.165.2.443-447.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]