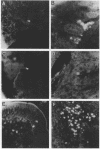
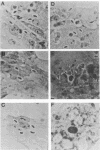
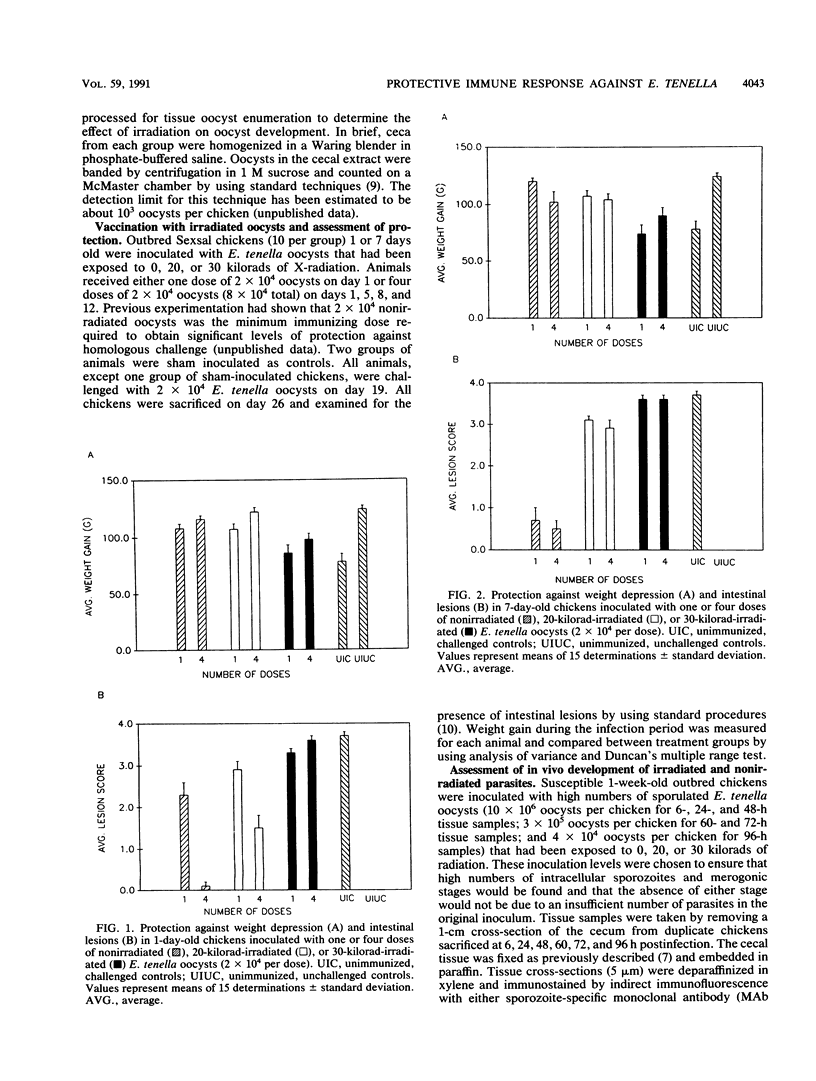
Abstract
Sporulated oocysts of the protozoan parasite Eimeria tenella were attenuated by exposure to various doses of X-radiation to inhibit intracellular replication and thus determine whether sporozoites alone can induce a protective immune response. Exposure to doses greater than 15-kilorads had a significant effect on development, as indicated by the absence of oocyst production in chickens infected with parasites treated with 20 or 30 kilorads of radiation. Infection with nonirradiated or 15-kilorad-exposed parasites led to either normal or reduced oocyst shedding. Equivalent protection was afforded chickens inoculated with a minimum immunizing dose of either nonirradiated or 20-kilorad-irradiated E. tenella oocysts. Immunofluorescence staining of cecal tissue from chickens inoculated with 10(7) nonirradiated or 20- or 30-kilorad-irradiated oocysts with stage-specific monoclonal antibodies showed no significant difference in sporozoite invasion between treatment groups. Normal merogonic development was observed at appropriate times (48, 60, 72, and 96 h) postinfection in chickens inoculated with nonirradiated oocysts. In contrast, irradiated parasites exhibited minimal merogonic development at 48 h postinfection. Furthermore, no merogonic stages were observed at times of otherwise peak merozoite development (60, 72, and 96 h) in cecal tissue from chickens inoculated with irradiated parasites. Infection of chicken cells with irradiated or nonirradiated parasites in vitro corroborated these findings and indicate that events early after sporozoite invasion induce a protective immune response against this parasite.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine P. C., Danforth H. D. Avian eimeria: invasion in foreign host birds and generation of partial immunity against coccidiosis. Avian Dis. 1990 Jan-Mar;34(1):196–202. [PubMed] [Google Scholar]
- Clyde D. F. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975 May;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Augustine P. C. Eimeria tenella: use of a monoclonal antibody in determining the intracellular fate of the refractile body organelles and the effect on in vitro development. Exp Parasitol. 1989 Jan;68(1):1–7. doi: 10.1016/0014-4894(89)90002-7. [DOI] [PubMed] [Google Scholar]
- Jeffers T. K., Long P. L. Eimeria tenella: immunogenicity of arrested sporozoites in chickens. Exp Parasitol. 1985 Oct;60(2):175–180. doi: 10.1016/0014-4894(85)90021-9. [DOI] [PubMed] [Google Scholar]
- Jenkins M. C., Augustine P. C., Barta J. R., Castle M. D., Danforth H. D. Development of resistance to coccidiosis in the absence of merogonic development using X-irradiated Eimeria acervulina oocysts. Exp Parasitol. 1991 Apr;72(3):285–293. doi: 10.1016/0014-4894(91)90148-p. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970 Aug;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kogut M. H., Lange C. Effect of lymphokine treatment on the invasion of cultured animal cells by Eimeria tenella. Lymphokine Res. 1988 Spring;7(1):31–44. [PubMed] [Google Scholar]
- Leathem W. D., Burns W. C. Effects of the immune chicken on the endogenous stages of Eimeria tenella. J Parasitol. 1967 Feb;53(1):180–185. [PubMed] [Google Scholar]
- Lillehoj H. S. Effects of immunosuppression on avian coccidiosis: cyclosporin A but not hormonal bursectomy abrogates host protective immunity. Infect Immun. 1987 Jul;55(7):1616–1621. doi: 10.1128/iai.55.7.1616-1621.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long P. L., Millard B. J. Eimeria: effect of meticlorpindol and methyl benzoquate on endogenous stages in the chicken. Exp Parasitol. 1968 Dec;23(3):331–338. doi: 10.1016/0014-4894(68)90025-8. [DOI] [PubMed] [Google Scholar]
- McDonald V., Wisher M. H., Rose M. E., Jeffers T. K. Eimeria tenella: immunological diversity between asexual generations. Parasite Immunol. 1988 Nov;10(6):649–660. doi: 10.1111/j.1365-3024.1988.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig R. S., Vanderberg J. P., Most H., Orton C. Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites. Nature. 1969 May 3;222(5192):488–489. doi: 10.1038/222488a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. S. Immunity of rats and mice following injection with 60Co irradiated Babesia rodhaini infected red cells. Parasitology. 1971 Apr;62(2):221–231. doi: 10.1017/s0031182000071468. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: T-lymphocyte- or B-lymphocyte-deficient animals. Infect Immun. 1979 Nov;26(2):630–637. doi: 10.1128/iai.26.2.630-637.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: stages of the life-cycle of Eimeria maxima which induce, and are affected by, the response of the host. Parasitology. 1976 Aug;73(1):25–57. doi: 10.1017/s0031182000051295. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Joysey H. S., Hesketh P., Grencis R. K., Wakelin D. Mediation of immunity to Eimeria vermiformis in mice by L3T4+ T cells. Infect Immun. 1988 Jul;56(7):1760–1765. doi: 10.1128/iai.56.7.1760-1765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Hesketh P. Gamma interferon controls Eimeria vermiformis primary infection in BALB/c mice. Infect Immun. 1989 May;57(5):1599–1603. doi: 10.1128/iai.57.5.1599-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Hesketh P. Interferon-gamma-mediated effects upon immunity to coccidial infections in the mouse. Parasite Immunol. 1991 Jan;13(1):63–74. doi: 10.1111/j.1365-3024.1991.tb00263.x. [DOI] [PubMed] [Google Scholar]
- Ryley J. F., Meade R., Hazelhurst J., Robinson T. E. Methods in coccidiosis research: separation of oocysts from faeces. Parasitology. 1976 Dec;73(3):311–326. doi: 10.1017/s0031182000046990. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Crane M. S., Murray P. K. Purification of Eimeria sporozoites by DE-52 anion exchange chromatography. J Protozool. 1984 Feb;31(1):181–183. doi: 10.1111/j.1550-7408.1984.tb04314.x. [DOI] [PubMed] [Google Scholar]
- Suhrbier A., Winger L. A., Castellano E., Sinden R. E. Survival and antigenic profile of irradiated malarial sporozoites in infected liver cells. Infect Immun. 1990 Sep;58(9):2834–2839. doi: 10.1128/iai.58.9.2834-2839.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. R., Mellouk S., Houghten R. A., Sedegah M., Kumar S., Good M. F., Berzofsky J. A., Miller L. H., Hoffman S. L. Cytotoxic T cells recognize a peptide from the circumsporozoite protein on malaria-infected hepatocytes. J Exp Med. 1990 Mar 1;171(3):763–773. doi: 10.1084/jem.171.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]