Abstract
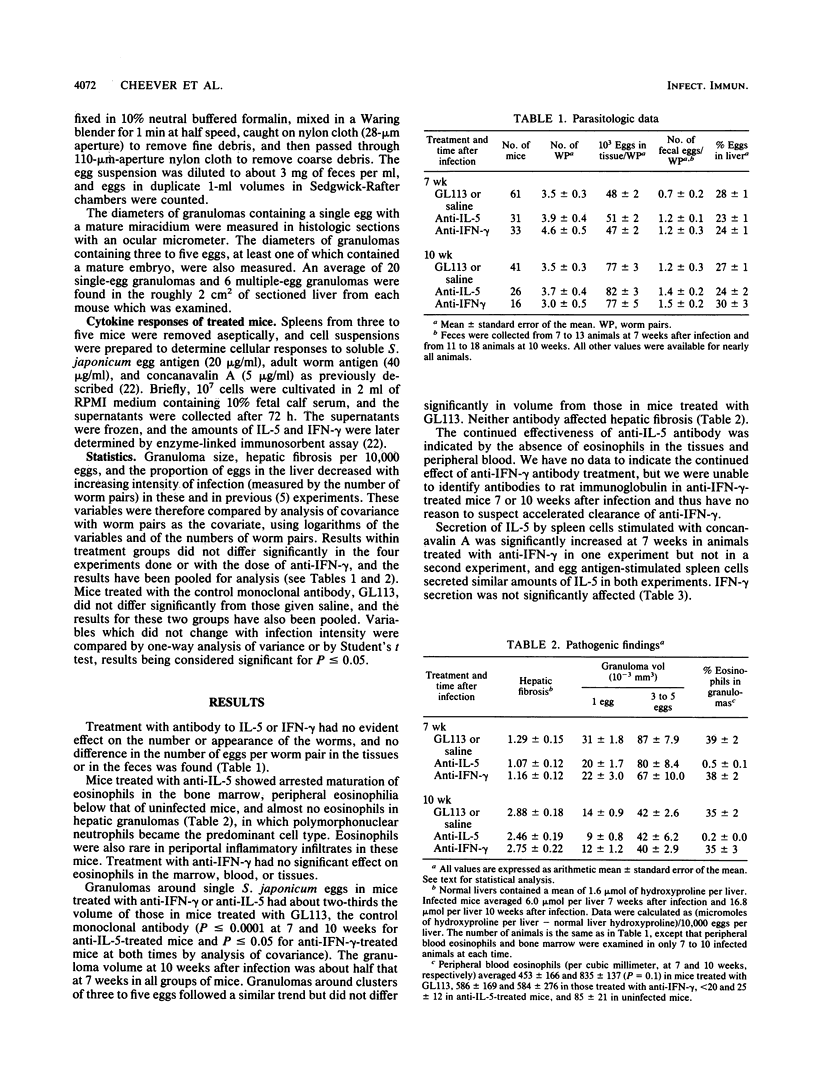
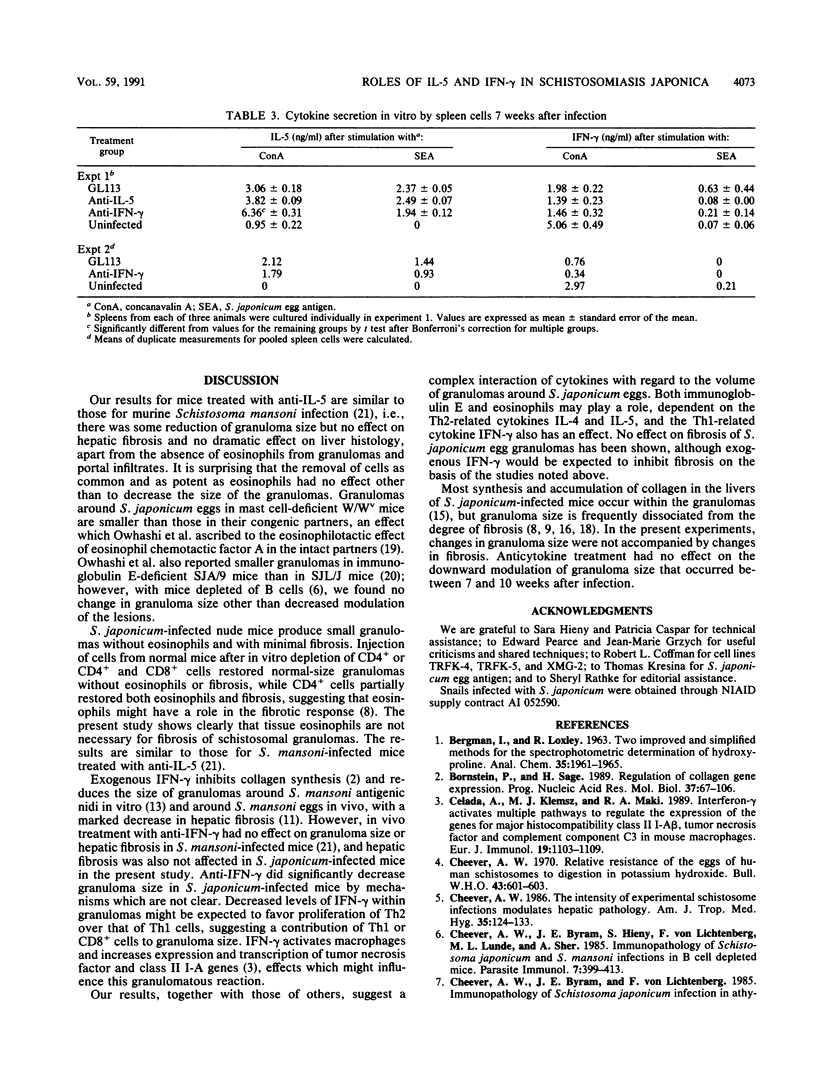
Schistosoma japonicum-infected mice were treated with antibodies to interleukin-5 (IL-5) or gamma interferon (IFN-gamma) from week 3 or 4 to week 10 of infection. Neither antibody affected egg production by the parasite, and neither had a consistent effect on the secretion of IFN-gamma or IL-5 cell-related cytokines by spleen cells from infected mice. Mice treated with antibody to murine IL-5 had only rare eosinophils in hepatic circumoval granulomas. Granulomas around single eggs were reduced in volume by a third, but hepatic fibrosis was unaffected. Treatment with antibody to murine IFN-gamma also reduced the size of granulomas and also did not affect hepatic fibrosis, which was measured as hydroxyproline. Our results, taken together with the studies of others, indicate that a complex interaction of cytokines affects granuloma size and that the size and fibrosis of granulomas are to some extent regulated independently.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P., Sage H. Regulation of collagen gene expression. Prog Nucleic Acid Res Mol Biol. 1989;37:67–106. doi: 10.1016/s0079-6603(08)60695-9. [DOI] [PubMed] [Google Scholar]
- Celada A., Klemsz M. J., Maki R. A. Interferon-gamma activates multiple pathways to regulate the expression of the genes for major histocompatibility class II I-A beta, tumor necrosis factor and complement component C3 in mouse macrophages. Eur J Immunol. 1989 Jun;19(6):1103–1109. doi: 10.1002/eji.1830190621. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., Hieny S., von Lichtenberg F., Lunde M. N., Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985 Jul;7(4):399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Deb S., Duvall R. H. Granuloma formation in Schistosoma japonicum infected nude mice: the effects of reconstitution with L3T4+ or Lyt2+ splenic cells. Am J Trop Med Hyg. 1989 Jan;40(1):66–71. doi: 10.4269/ajtmh.1989.40.66. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Duvall R. H., Hallack T. A., Jr Differences in hepatic fibrosis and granuloma size in several strains of mice infected with Schistosoma japonicum. Am J Trop Med Hyg. 1984 Jul;33(4):602–607. doi: 10.4269/ajtmh.1984.33.602. [DOI] [PubMed] [Google Scholar]
- Cheever A. W. Relative resistance of the eggs of human schistosomes to digestion in potassium hydroxide. Bull World Health Organ. 1970;43(4):601–603. [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W. The intensity of experimental schistosome infections modulates hepatic pathology. Am J Trop Med Hyg. 1986 Jan;35(1):124–133. doi: 10.4269/ajtmh.1986.35.124. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Takahashi S., Giambrone M. A., van der Meide P. H., Schellekens H., Biempica L., Zern M. A. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989 Nov;10(5):795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Lammie P. J., Phillips S. M., Linette G. P., Michael A. I., Bentley A. G. In vitro granuloma formation using defined antigenic nidi. Ann N Y Acad Sci. 1986;465:340–350. doi: 10.1111/j.1749-6632.1986.tb18509.x. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Griffin A., Kresina T. F. Dynamics of collagen accumulation and polymorphism in murine Schistosoma japonicum. Gastroenterology. 1985 Sep;89(3):617–624. doi: 10.1016/0016-5085(85)90459-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Kresina T. F. Immunoregulation of hepatic fibrosis in murine schistosomiasis japonica. J Infect Dis. 1989 Apr;159(4):798–801. doi: 10.1093/infdis/159.4.798. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981 May 15;60(2):251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Stavitsky A. B. Mechanisms of in vivo modulation of granulomatous inflammation in murine schistosomiasis japonicum. Infect Immun. 1986 May;52(2):513–518. doi: 10.1128/iai.52.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owhashi M., Horii Y., Ikeda T., Maruyama H., Abe T., Nawa Y. Importance of mast-cell-derived eosinophil chemotactic factor A on granuloma formation in murine Schistosomiasis japonica: evaluation using mast-cell-deficient W/WV mice. Int Arch Allergy Appl Immunol. 1990;92(1):64–68. doi: 10.1159/000235226. [DOI] [PubMed] [Google Scholar]
- Owhashi M., Nawa Y., Watanabe N. Granulomatous response in selective IgE-deficient SJA/9 mice infected with Schistosoma japonicum. Int Arch Allergy Appl Immunol. 1989;90(3):310–312. doi: 10.1159/000235044. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. H., Macedonia J., Sher A., Pearce E., Cheever A. W. Dynamic analysis of splenic Th1 and Th2 lymphocyte functions in mice infected with Schistosoma japonicum. Infect Immun. 1991 Sep;59(9):2934–2940. doi: 10.1128/iai.59.9.2934-2940.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


