Abstract
To determine whether oxidative metabolic products of phagocytic cells are present in the middle ear during experimental pneumococcal otitis media, we measured the concentration of myeloperoxidase (MPO) in middle ear fluid (MEF) and the capacity of neutrophils isolated from MEF and peripheral blood to produce MPO and superoxide anion (O2-) after in vitro stimulation. Free MPO in MEF was significantly increased 24 and 48 h after either viable or nonviable pneumococci were inoculated into the middle ear. In vitro-stimulated production of MPO and O2- from middle ear neutrophils was significantly less than that from peripheral blood neutrophils 24 h after nonviable pneumococci were inoculated but similar to it after 48 h. Twenty-four hours after viable pneumococci were inoculated, middle ear neutrophils stimulated in vitro produced less MPO but the same amount of O2- as did blood neutrophils. Oxidative metabolic products, therefore, are released from phagocytic cells into the MEF during pneumococcal otitis media, and future studies will need to define the contribution of these products to acute and chronic middle ear tissue injury.
Full text
PDF
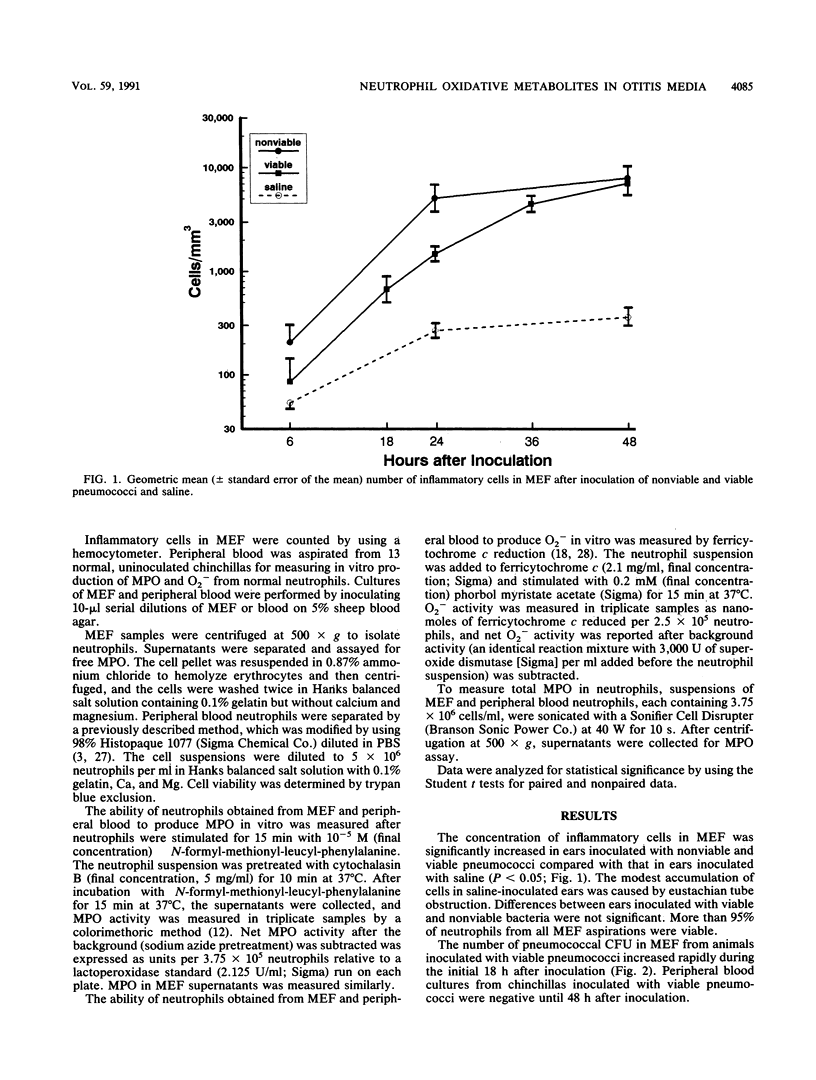
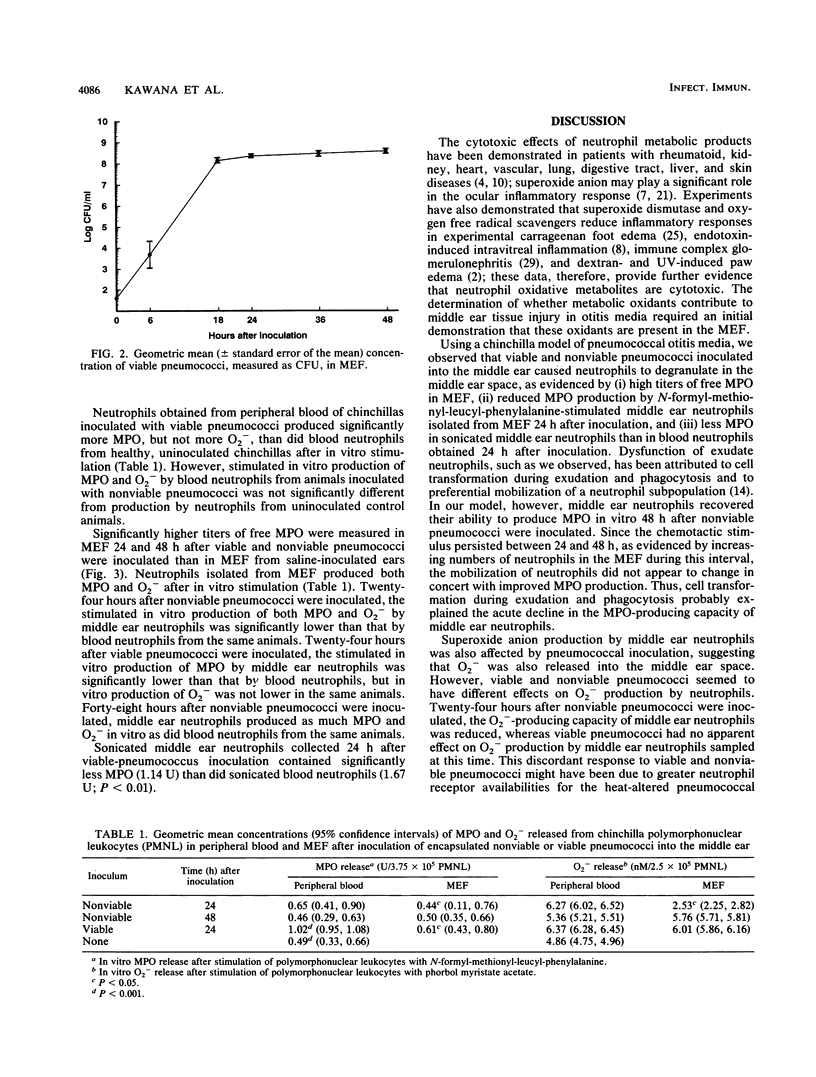
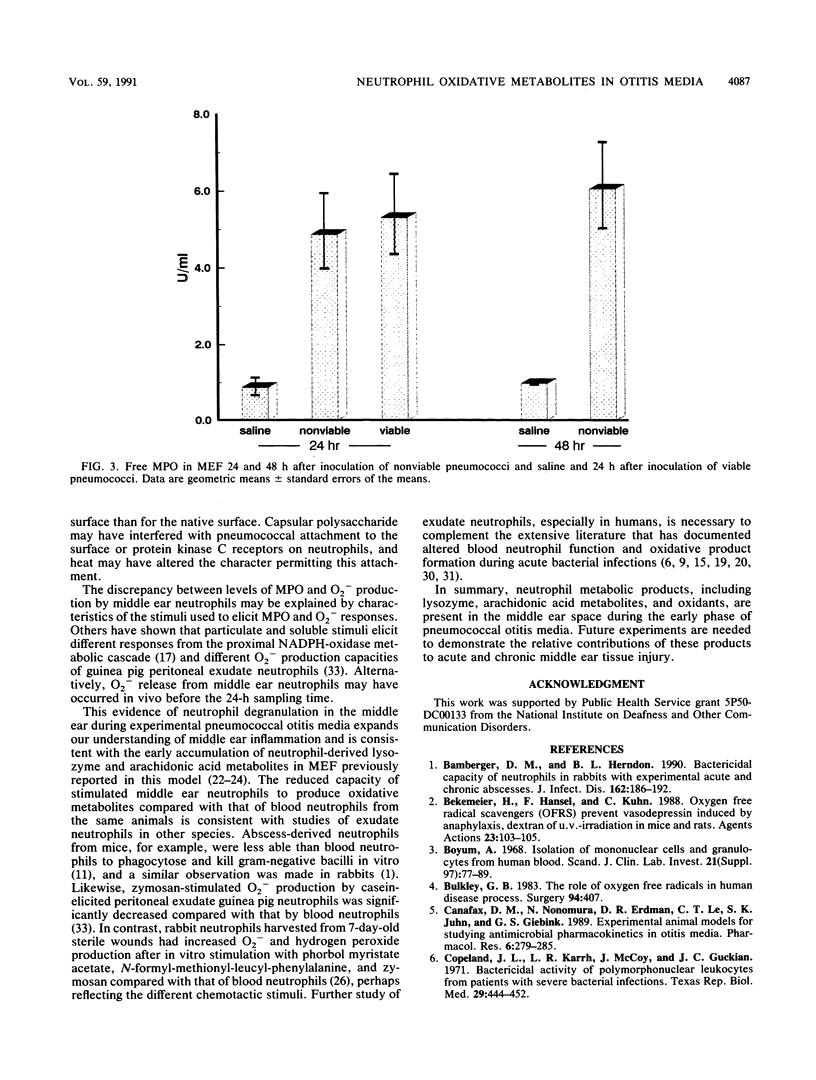

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger D. M., Herndon B. L. Bactericidal capacity of neutrophils in rabbits with experimental acute and chronic abscesses. J Infect Dis. 1990 Jul;162(1):186–192. doi: 10.1093/infdis/162.1.186. [DOI] [PubMed] [Google Scholar]
- Bekemeier H., Hänsel F., Kuhn C. Oxygen free radical scavengers (OFRS) prevent vasodepression induced by anaphylaxis, dextran or u.v.-irradiation in mice and rats. Agents Actions. 1988 Feb;23(1-2):103–105. doi: 10.1007/BF01967205. [DOI] [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Canafax D. M., Nonomura N., Erdmann G. R., Le C. T., Juhn S. K., Giebink G. S. Experimental animal models for studying antimicrobial pharmacokinetics in otitis media. Pharm Res. 1989 Apr;6(4):279–285. doi: 10.1023/a:1015938205892. [DOI] [PubMed] [Google Scholar]
- Elgebaly S. A., Herkert N., O'Rourke J., Kreutzer D. L. Characterization of neutrophil and monocyte specific chemotactic factors derived from the cornea in response to hydrogen peroxide injury. Am J Pathol. 1987 Jan;126(1):40–50. [PMC free article] [PubMed] [Google Scholar]
- Fleisher L. N., Ferrell J. B., Olson N. C., McGahan M. C. Dimethylthiourea inhibits the inflammatory response to intravitreally-injected endotoxin. Exp Eye Res. 1989 Apr;48(4):561–567. doi: 10.1016/0014-4835(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Giebink G. S., Mills E. L., Huff J. S., Cates K. L., Juhn S. K., Quie P. G. Polymorphonuclear leukocyte dysfunction in children with recurrent otitis media. J Pediatr. 1979 Jan;94(1):13–18. doi: 10.1016/s0022-3476(79)80342-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nulsen M. F., McDonald P. J., Finlay-Jones J. J. Neutrophil activity in abscess-bearing mice: comparative studies with neutrophils isolated from peripheral blood, elicited peritoneal exudates, and abscesses. Infect Immun. 1986 Mar;51(3):936–941. doi: 10.1128/iai.51.3.936-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Lalchev S., Karchev T. Phagocytic activity and bactericidal capacity of polymorphonuclear leukocytes in children with recurrent otitis media. Int J Pediatr Otorhinolaryngol. 1985 Nov;10(2):165–170. doi: 10.1016/s0165-5876(85)80029-x. [DOI] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I., Tringale S. M., Tauber A. I. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986 Nov 1;137(9):2925–2929. [PubMed] [Google Scholar]
- McCall C. E., Caves J., Cooper R., DeChatlet L. Functional characteristics of human toxic neutrophils. J Infect Dis. 1971 Jul;124(1):68–75. doi: 10.1093/infdis/124.1.68. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Reed W. P., Palmer D. L., Bolin R. B., Davis A. T., Quie P. G. A transient defect in leukocytic bactericidal capacity. Clin Immunol Immunopathol. 1973 Jul;1(4):523–532. doi: 10.1016/0090-1229(73)90008-1. [DOI] [PubMed] [Google Scholar]
- Mittag T. Role of oxygen radicals in ocular inflammation and cellular damage. Exp Eye Res. 1984 Dec;39(6):759–769. doi: 10.1016/0014-4835(84)90075-7. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Juhn S. K., Harada T., Aeppli D. Pathophysiology of Streptococcus pneumoniae otitis media: kinetics of the middle ear biochemical and cytologic host responses. Ann Otol Rhinol Laryngol. 1991 Mar;100(3):236–243. doi: 10.1177/000348949110000313. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Zelterman D., Harada T., Juhn S. K. Early biochemical events in pneumococcal otitis media: arachidonic acid metabolites in middle ear fluid. Ann Otol Rhinol Laryngol. 1991 May;100(5 Pt 1):385–388. doi: 10.1177/000348949110000507. [DOI] [PubMed] [Google Scholar]
- Nonomura N., Giebink G. S., Zelterman D., Harada T., Juhn S. K. Middle ear fluid lysozyme source in experimental pneumococcal otitis media. Ann Otol Rhinol Laryngol. 1991 Jul;100(7):593–596. doi: 10.1177/000348949110000715. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem Pharmacol. 1976 Jul 1;25(13):1465–1472. [PubMed] [Google Scholar]
- Paty P. B., Graeff R. W., Waldman F. M., Hunt T. K., Mathes S. J. Biologic priming of neutrophils in subcutaneous wounds. Arch Surg. 1988 Dec;123(12):1509–1513. doi: 10.1001/archsurg.1988.01400360079013. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Rahman M. A., Emancipator S. S., Sedor J. R. Hydroxyl radical scavengers ameliorate proteinuria in rat immune complex glomerulonephritis. J Lab Clin Med. 1988 Nov;112(5):619–626. [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Neutrophil granulocyte function in bacterial infections. Lancet. 1972 Oct 7;2(7780):727–730. doi: 10.1016/s0140-6736(72)92022-3. [DOI] [PubMed] [Google Scholar]
- Solomkin J. S., Bauman M. P., Nelson R. D., Simmons R. L. Neutrophils dysfunction during the course of intra-abdominal infection. Ann Surg. 1981 Jul;194(1):9–17. doi: 10.1097/00000658-198107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate R. M., Repine J. E. Neutrophils and the adult respiratory distress syndrome. Am Rev Respir Dis. 1983 Sep;128(3):552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Lew P. D., Cohen H. J., Waldvogel F. A. Comparative superoxide-generating system of granulocytes from blood and peritoneal exudates. Infect Immun. 1984 Dec;46(3):625–630. doi: 10.1128/iai.46.3.625-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



