Abstract
An experimental skin lesion induced in rabbits by the bite of infected adult Ixodes dammini showed dense dermal interstitial inflammatory cell infiltrates composed of mononuclear cells (histiocytes and lymphocytes) and granulocytes. The prevalence of phagocytic cells in this experimental lesion motivated a study on the interactions of macrophages and neutrophils with Lyme disease spirochetes. Interactions as measured by uptake of radiolabeled spirochetes and by indirect immunofluorescence were enhanced by opsonization of spirochetes with immune serum and not significantly decreased by heat inactivation of the same. Phagocytosis was inhibited by treatment of cells with Cytochalasin B. Adherence of opsonized spirochetes to neutrophils was decreased by blocking Fc receptors with heat-aggregated IgG, suggesting an important role for this receptor.
Full text
PDF



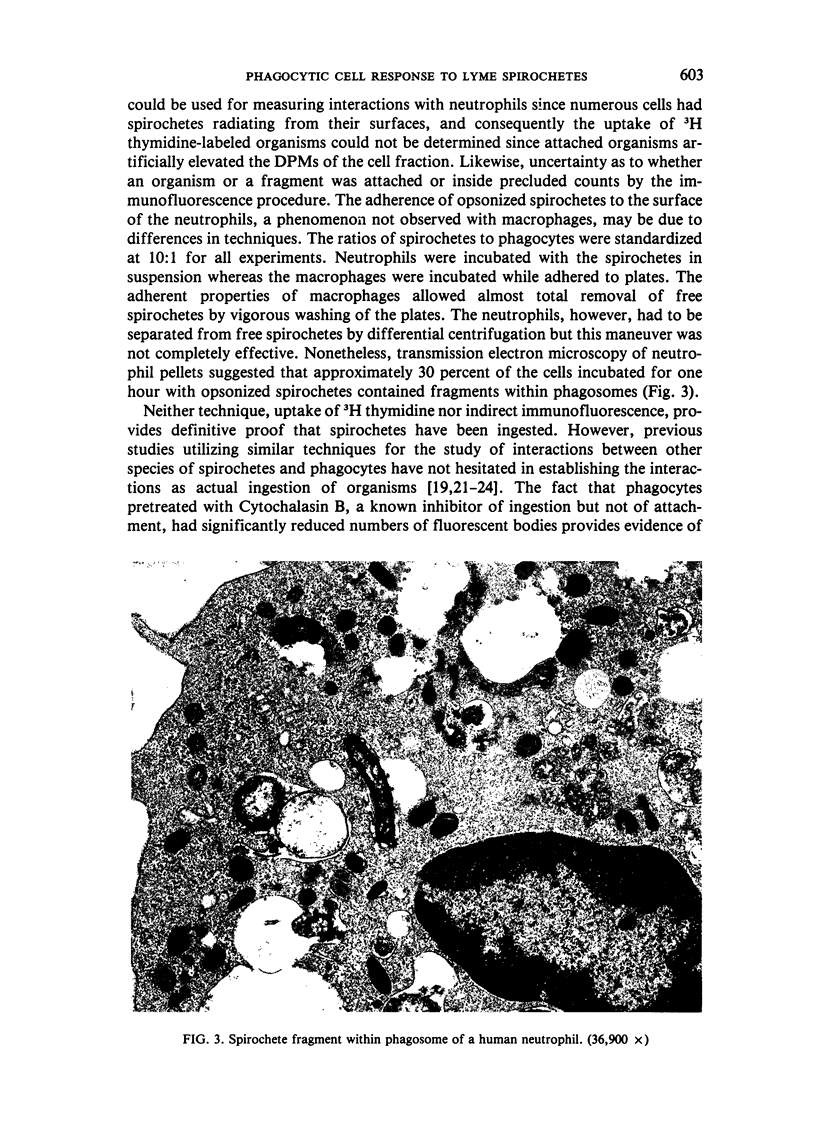


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Berenberg J. L., Ward P. A., Sonenshine D. E. Tick-bite injury: mediation by a complement-derived chemotictic chemotactic factor. J Immunol. 1972 Sep;109(3):451–456. [PubMed] [Google Scholar]
- Berger B. W., Clemmensen O. J., Ackerman A. B. Lyme disease is a spirochetosis. A review of the disease and evidence for its cause. Am J Dermatopathol. 1983 Apr;5(2):111–124. [PubMed] [Google Scholar]
- Bosler E. M., Coleman J. L., Benach J. L., Massey D. A., Hanrahan J. P., Burgdorfer W., Barbour A. G. Natural Distribution of the Ixodes dammini spirochete. Science. 1983 Apr 15;220(4594):321–322. doi: 10.1126/science.6836274. [DOI] [PubMed] [Google Scholar]
- Brossard M., Fivaz V. Ixodes ricinus L.: mast cells, basophils and eosinophils in the sequence of cellular events in the skin of infested or re-infested rabbits. Parasitology. 1982 Dec;85(Pt 3):583–592. doi: 10.1017/s0031182000056365. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Knapp F. W. Amblyomma americanum: sequential histological analysis of adult feeding sites on guinea pigs. Exp Parasitol. 1980 Jun;49(3):303–318. doi: 10.1016/0014-4894(80)90067-3. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Knapp F. W. Amblyomma americanum: sequential histological analysis of larval and nymphal feeding sites on guinea pigs. Exp Parasitol. 1980 Apr;49(2):188–205. doi: 10.1016/0014-4894(80)90116-2. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Butler T., Aikawa M., Habte-Michael A., Wallace C. Phagocytosis of Borrelia recurrentis by blood polymorphonuclear leukocytes is enhanced by antibiotic treatment. Infect Immun. 1980 Jun;28(3):1009–1013. doi: 10.1128/iai.28.3.1009-1013.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T., Spagnuolo P. J., Goldsmith G. H., Aikawa M. Interaction of Borrelia spirochetes with human mononuclear leukocytes causes production of leukocytic pyrogen and thromboplastin. J Lab Clin Med. 1982 May;99(5):709–721. [PubMed] [Google Scholar]
- Hardin J. A., Walker L. C., Steere A. C., Trumble T. C., Tung K. S., Williams R. C., Jr, Ruddy S., Malawista S. E. Circulating immune complexes in Lyme arthritis. Detection by the 125I-C1q binding, C1q solid phase, and Raji cell assays. J Clin Invest. 1979 Mar;63(3):468–477. doi: 10.1172/JCI109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Krinsky W. L., Brown S. J., Askenase P. W. Ixodes dammini: induced skin lesions in guinea pigs and rabbits compared to erythema chronicum migrans in patients with lyme arthritis. Exp Parasitol. 1982 Jun;53(3):381–395. doi: 10.1016/0014-4894(82)90081-9. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Miller J. N. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J Immunol. 1978 Nov;121(5):2014–2024. [PubMed] [Google Scholar]
- Malawista S. E., Gee J. B., Bensch K. G. Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J Biol Med. 1971 Dec;44(3):286–300. [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Hague-Park M., Gyorkey F., Anderson D. C., Baughn R. E. The interaction between Treponema pallidum and human polymorphonuclear leukocytes. J Infect Dis. 1983 Jan;147(1):77–86. doi: 10.1093/infdis/147.1.77. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. The participation of macrophages and macrophage cell lines in the activation of T lymphocytes by mitogens. Immunol Rev. 1978;40:102–135. doi: 10.1111/j.1600-065x.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Sell S., Gamboa D., Baker-Zander S. A., Lukehart S. A., Miller J. N. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. J Invest Dermatol. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- Spagnuolo P. J., Butler T., Bloch E. H., Santoro C., Tracy J. W., Johnson R. C. Opsonic requirements for phagocytosis of Borrelia hermsii by human polymorphonuclear leukocytes. J Infect Dis. 1982 Mar;145(3):358–364. doi: 10.1093/infdis/145.3.358. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Bartenhagen N. H., Craft J. E., Hutchinson G. J., Newman J. H., Rahn D. W., Sigal L. H., Spieler P. N., Stenn K. S., Malawista S. E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983 Jul;99(1):76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Hardin J. A., Ruddy S., Askenase W., Andiman W. A. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977 Jun;86(6):685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]