Abstract
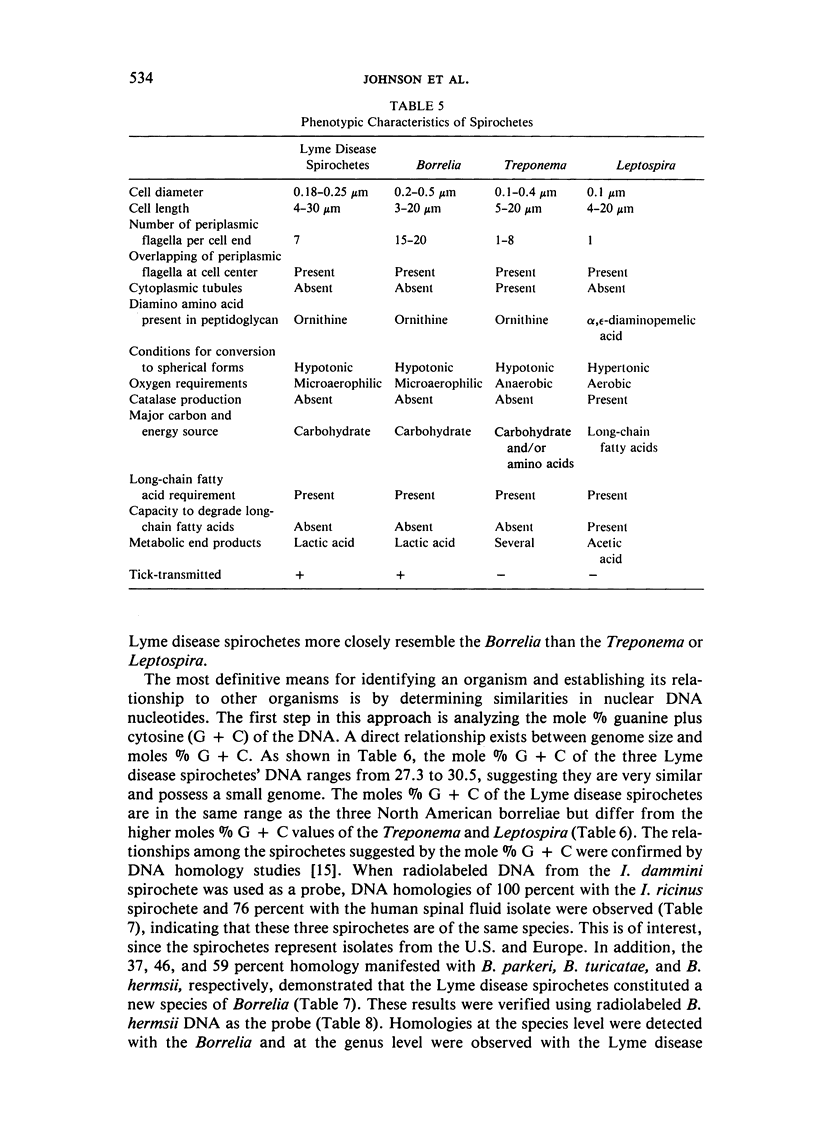
Morphology, physiology, and DNA nucleotide composition of Lyme disease spirochetes, Borrelia, Treponema, and Leptospira were compared. Morphologically, Lyme disease spirochetes resemble Borrelia. They lack cytoplasmic tubules present in Treponema, and have more than one periplasmic flagellum per cell end and lack the tight coiling which are characteristic of Leptospira. Lyme disease spirochetes are also similar to Borrelia in being microaerophilic, catalase-negative bacteria. They utilize carbohydrates such as glucose as their major carbon and energy sources and produce lactic acid. Long-chain fatty acids are not degraded but are incorporated unaltered into cellular lipids. The diamino amino acid present in the peptidoglycan is ornithine. The mole % guanine plus cytosine values for Lyme disease spirochete DNA were 27.3-30.5 percent. These values are similar to the 28.0-30.5 percent for the Borrelia but differed from the values of 35.3-53 percent for Treponema and Leptospira. DNA reannealing studies demonstrated that Lyme disease spirochetes represent a new species of Borrelia, exhibiting a 31-59 percent DNA homology with the three species of North American borreliae. In addition, these studies showed that the three Lyme disease spirochetes comprise a single species with DNA homologies ranging from 76-100 percent. The three North American borreliae also constitute a single species, displaying DNA homologies of 75-95 percent. Lyme disease spirochetes and Borrelia exhibited little or no DNA homology (0-2 percent) with the Treponema or Leptospira. Plasmids were present in the three Lyme disease spirochetes and the three North American borreliae.
Full text
PDF


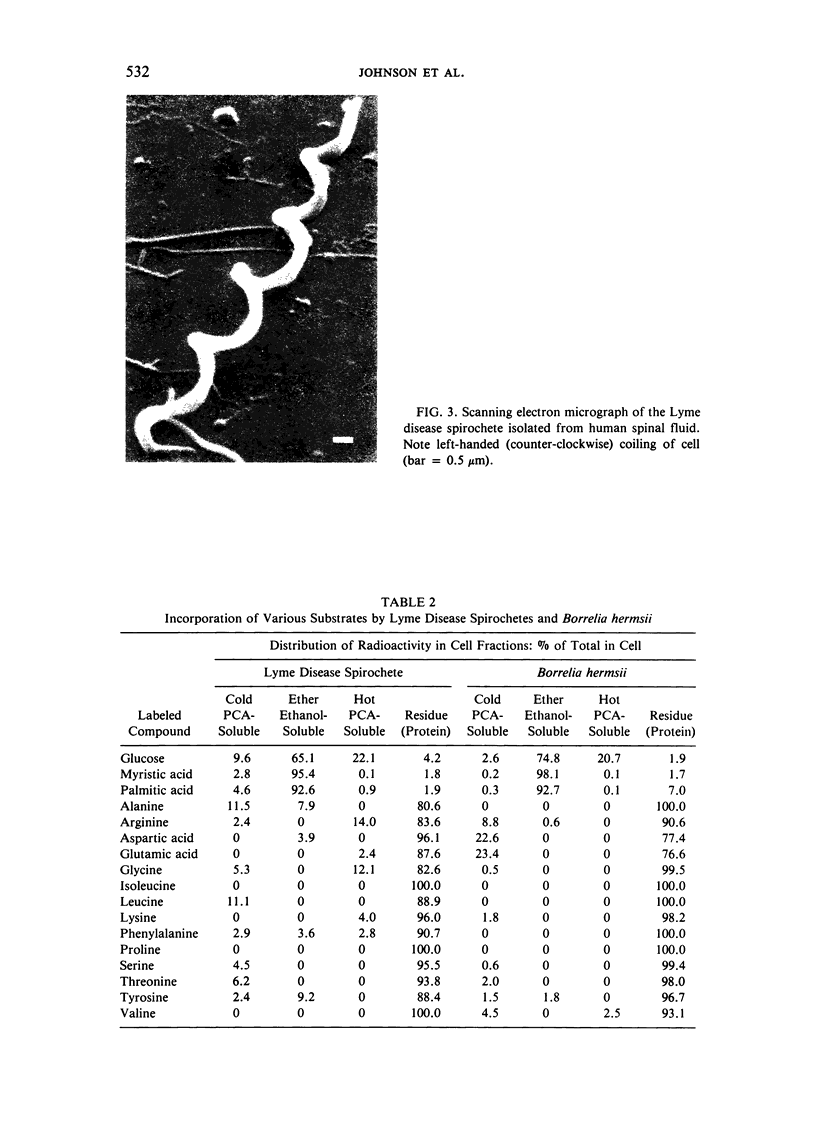




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auran N. E., Johnson R. C., Ritzi D. M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972 Jun;5(6):968–975. doi: 10.1128/iai.5.6.968-975.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Butler T., Hazen P., Wallace C. K., Awoke S., Habte-Michael A. Infection with Borrelia recurrentis: pathogenesis of fever and petechiae. J Infect Dis. 1979 Nov;140(5):665–675. doi: 10.1093/infdis/140.5.665. [DOI] [PubMed] [Google Scholar]
- Hayes S. F., Burgdorfer W., Barbour A. G. Bacteriophage in the Ixodes dammini spirochete, etiological agent of Lyme disease. J Bacteriol. 1983 Jun;154(3):1436–1439. doi: 10.1128/jb.154.3.1436-1439.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. Cultivation of Borrelia hermsi. Science. 1971 Jul 30;173(3995):443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- Livermore B. P., Bey R. F., Johnson R. C. Lipid metabolism of Borrelia hermsi. Infect Immun. 1978 Apr;20(1):215–220. doi: 10.1128/iai.20.1.215-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Plasmid DNA in Treponema pallidum (Nichols): potential for antibiotic resistance by syphilis bacteria. Science. 1981 Jul 31;213(4507):553–555. doi: 10.1126/science.6264606. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stepan D. E., Johnson R. C. Helical conformation of Treponema pallidum (Nichols strain), Treponema paraluis-cuniculi, Treponema denticola, Borrelia turicatae, and unidentified oral spirochetes. Infect Immun. 1981 May;32(2):937–940. doi: 10.1128/iai.32.2.937-940.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]