Abstract
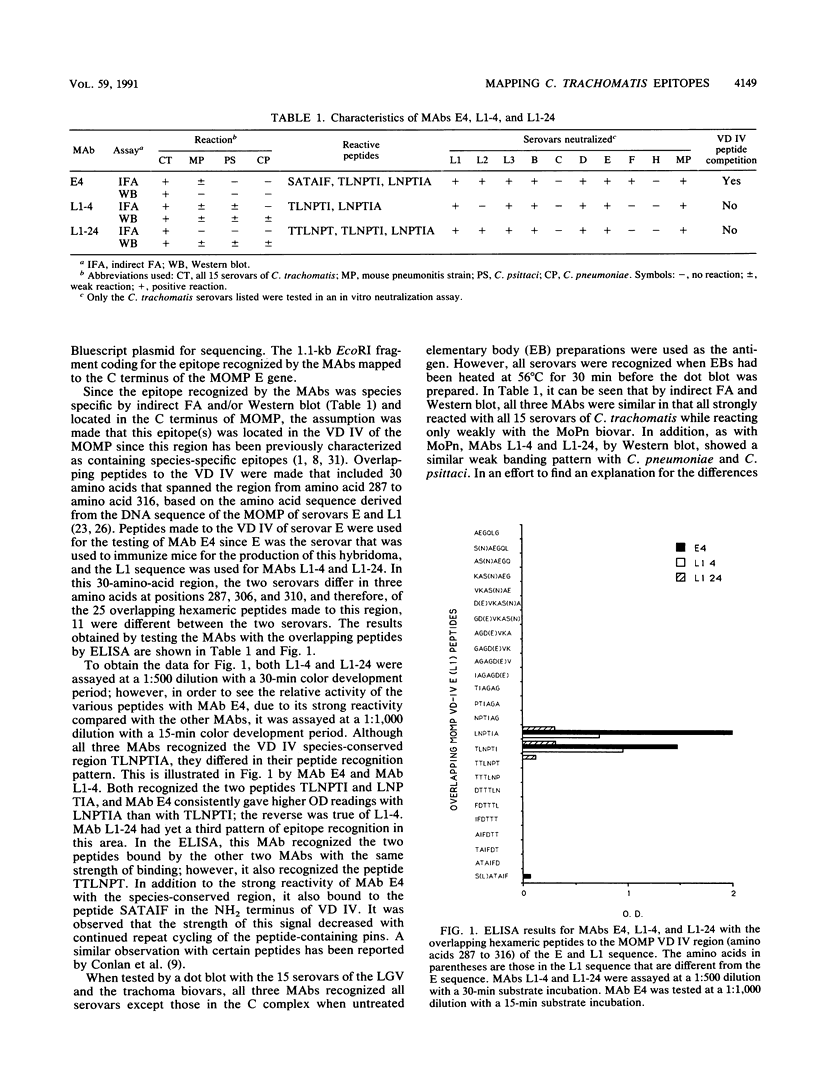
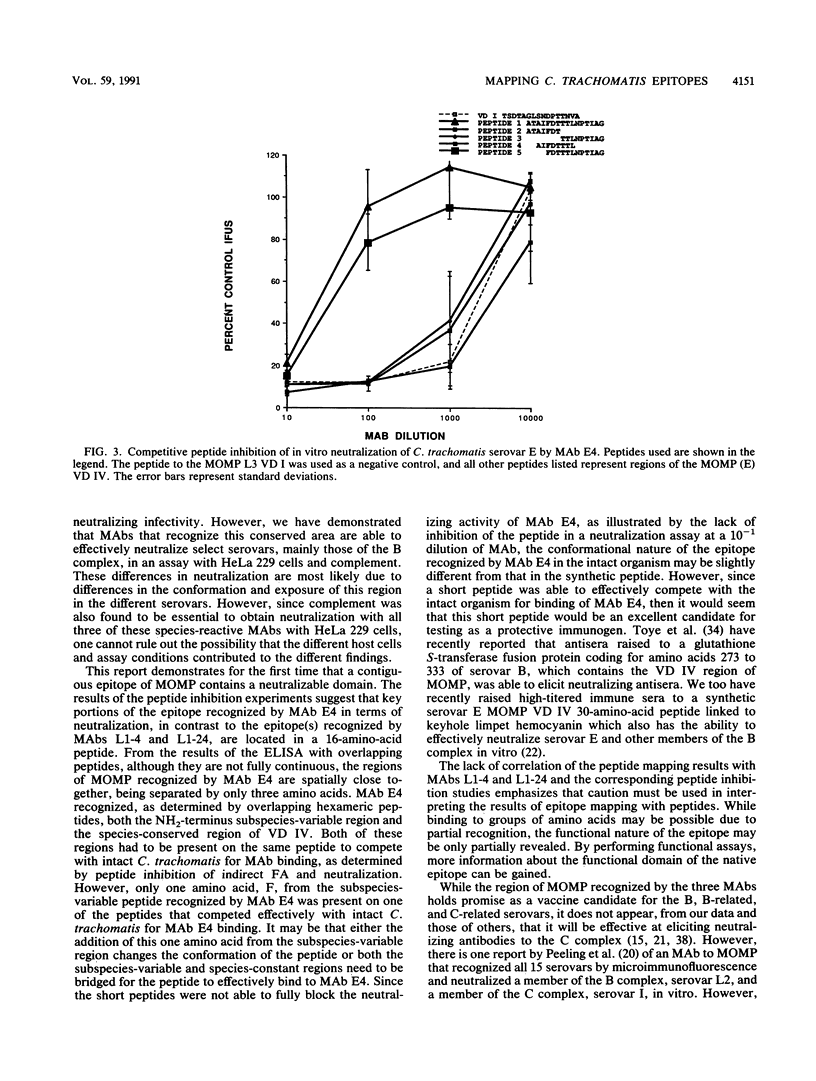
Three monoclonal antibodies (MAbs), E4, L1-4, and L1-24, to the major outer membrane protein (MOMP) of Chlamydia trachomatis were identified that neutralized in vitro the infectivity of members of the B- and C-related complex as well as the mouse pneumonitis strain. MAbs L1-4, L1-24, and E4 gave a strong signal in an indirect immunofluorescence assay and/or Western immunoblot with all serovars of the lymphogranuloma venereum and trachoma biovars and a weak signal with the mouse biovar. In addition, C. psittaci and C. pneumoniae were also weakly recognized by MAbs L1-4 and L1-24. As determined by the technique of pneumoniae were also weakly recognized by MAbs L1-4 and L1-24. As determined by by the technique of overlapping peptides, all three MAbs showed reactivity to variable domain (VD) IV of MOMP. While all three MAbs had different recognition patterns, all strongly bound to the peptides TLNPTI and LNPTIA within the species-conserved region of VD IV. MAb E4 also recognized the peptide SATAIF in the subspecies region of VD IV. Peptides corresponding to VD IV of MOMP were synthesized and used in competitive inhibition experiments to determine the functional location of the epitope recognized by these three MAbs. Both the serological and neutralizing activities of MAb E4 were inhibited by the peptides ATAIFDTTTLNPTIAG and FDTTTLNPTIAG; however, none of the peptides made to the VD IV region blocked the neutralizing activity of MAbs L1-4 and L1-24. Therefore, the neutralizable domain of the epitope recognized by MAb E4 is contiguous and may be an important candidate for inclusion in a subunit vaccine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenfanger J., MacDonald A. B. The role of immunoglobulin in the neutralization of trachoma infectivity. J Immunol. 1974 Nov;113(5):1607–1617. [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson E. J., Peterson E. M., de la Maza L. M. Cloning and characterization of a Chlamydia trachomatis L3 DNA fragment that codes for an antigenic region of the major outer membrane protein and specifically hybridizes to the C- and C-related-complex serovars. Infect Immun. 1989 Feb;57(2):487–494. doi: 10.1128/iai.57.2.487-494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. L., Pannetier C., Jaulin C., Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. doi: 10.1093/nar/18.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., Clarke I. N., Ward M. E. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species- and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol. 1988 Sep;2(5):673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Conlan J. W., Kajbaf M., Clarke I. N., Chantler S., Ward M. E. The major outer membrane protein of Chlamydia trachomatis: critical binding site and conformation determine the specificity of antibody binding to viable chlamydiae. Mol Microbiol. 1989 Mar;3(3):311–318. doi: 10.1111/j.1365-2958.1989.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Mason T. J., Rodda S. J. Cognitive features of continuous antigenic determinants. J Mol Recognit. 1988 Feb;1(1):32–41. doi: 10.1002/jmr.300010107. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Tan T. W., Baxter S., Inglis N. F., Dunbar S. Sequence analysis of the major outer membrane protein gene of an ovine abortion strain of Chlamydia psittaci. FEMS Microbiol Lett. 1989 Nov;53(1-2):153–158. doi: 10.1016/0378-1097(89)90383-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucero M. E., Kuo C. C. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect Immun. 1985 Nov;50(2):595–597. doi: 10.1128/iai.50.2.595-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J. In defense of mucosal surfaces. Development of novel vaccines for IgA responses protective at the portals of entry of microbial pathogens. Infect Dis Clin North Am. 1990 Jun;4(2):315–341. [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Melgosa M., Kuo C. C., Campbell L. A. Sequence analysis of the major outer membrane protein gene of Chlamydia pneumoniae. Infect Immun. 1991 Jun;59(6):2195–2199. doi: 10.1128/iai.59.6.2195-2199.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., Schachter J., de la Maza L. M. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990 Mar;23(2):144–148. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., de la Maza L. M. The major outer membrane protein nucleotide sequence of Chlamydia trachomatis, serovar E. Nucleic Acids Res. 1990 Jun 11;18(11):3414–3414. doi: 10.1093/nar/18.11.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Tse P., Gastaldi C., Stone S. C., de la Maza L. M. Comparison of a single-antigen microimmunofluorescence assay and inclusion fluorescent-antibody assay for detecting chlamydial antibodies and correlation of the results with neutralizing ability. J Clin Microbiol. 1989 Feb;27(2):350–352. doi: 10.1128/jcm.27.2.350-352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toye B., Zhong G. M., Peeling R., Brunham R. C. Immunologic characterization of a cloned fragment containing the species-specific epitope from the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1990 Dec;58(12):3909–3913. doi: 10.1128/iai.58.12.3909-3913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Schachter J. Role of cell-mediated immunity in chlamydial infection: implications for ocular immunity. Rev Infect Dis. 1985 Nov-Dec;7(6):754–759. doi: 10.1093/clinids/7.6.754. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]


